
Sulfobutyl Beta Cyclodextrin Sodium CAS NO 182410-00-0
CAS No.: 182410-00-0
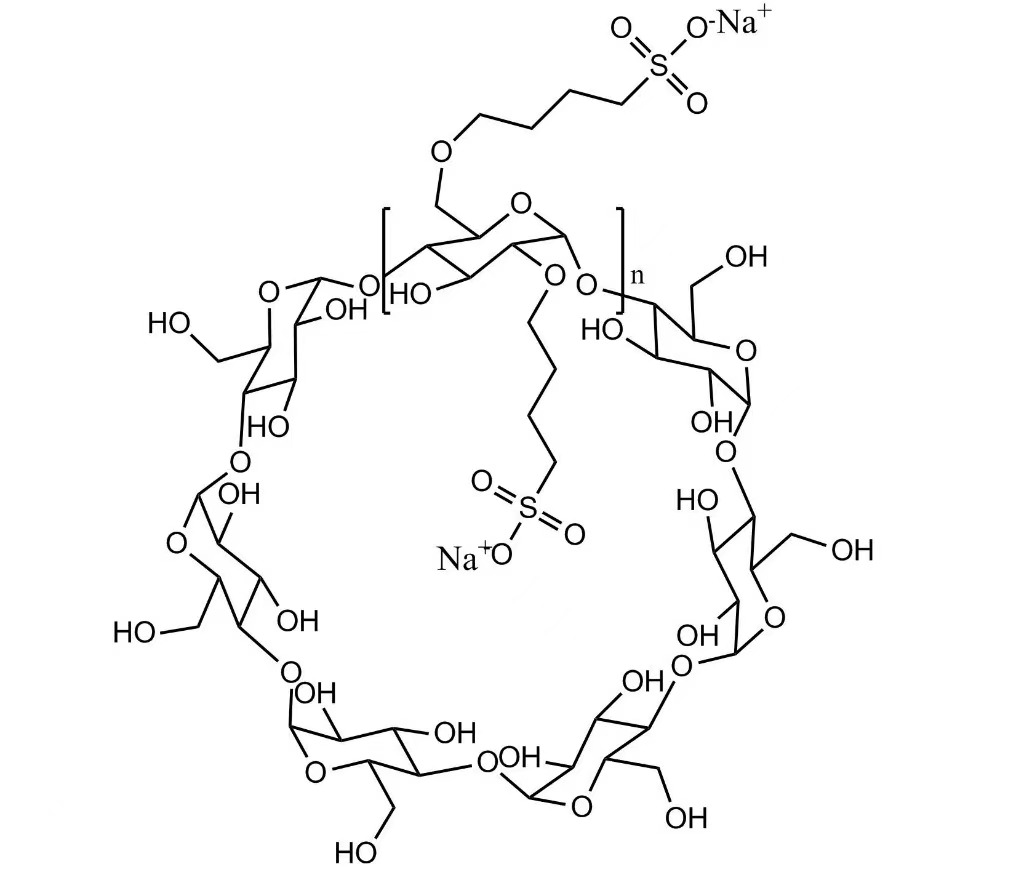
Molecular Formula: C42H70-nO35·(C4H8SO3Na)n
Grade: Injection Grade
Executive Standard: USP / EP / Enterprise Standard
Application Area: Pharmaceutical Use
Packaging: 500 g/bag; 1 kg/bag; 10 kg/bag or drum; customized packaging available
Sulfobutyl Ether Beta Cyclodextrin Sodium (Injection Grade) is a high-performance anionic cyclodextrin derivative produced by Xi'an DELI Biochemical Industry Co., Ltd.. It is widely used as a pharmaceutical excipient to improve drug solubility, stability, and safety in various dosage forms.
This product forms stable non-covalent inclusion complexes with active pharmaceutical ingredients, especially nitrogen-containing drugs, helping to enhance bioavailability, reduce toxicity, and mask unpleasant odor or taste.
Sulfobutyl Ether Beta Cyclodextrin Sodium has been successfully applied in injectable, oral, nasal, and ophthalmic formulations and is recognized as a safer alternative to traditional beta-cyclodextrin derivatives.
Product Name: Sulfobutyl Ether Beta Cyclodextrin Sodium
CAS No.: 182410-00-0
Molecular Formula: C42H70-nO35·(C4H8SO3Na)n
Grade: Injection Grade
Executive Standard: USP / EP / Enterprise Standard
Application Area: Pharmaceutical Use
Packaging: 500 g/bag; 1 kg/bag; 10 kg/bag or drum; customized packaging available
|
Description and Solubility/ see details in the USP Monograph |
White to off-white,amorphous powder.Freely soluble in water;sparingly soluble in methanol;practically insoluble in ethanol,in n-hexane,in 1-butanol,in acetonitrile,in 2-propanol,and in ethylacetate |
|
Identification A/IR; USP<197K> |
Complies with SBECD reference |
|
Identification B/(Assay method)HPLC; USP<621> |
tR of the major peak complies with SBECD reference |
|
Identification C/CE; USP<1053> |
It meets the requirements of the test for Average Degree of Substitution. |
|
Identification D/USP<191> |
Positive test for sodium |
|
Assay/HPLC;USP<621> |
95.0%-105.0% on the anhydrous basis |
|
Limit of Beta Cyclodextrin(Betadex)/IC; USP<621>;USP<1065> |
NMT 0.1% |
|
Limit of 1,4-Butane Sultone /GC;USP<621>, |
NMT 0.5ppm |
|
Limit of Sodium chloride /IC; USP<621>;USP<1065> |
NMT 0.2% |
|
Limit of 4-Hydroxybutane-1-sulfonic acid /IC;USP<621>;USP<1065> |
NMT 0.09% |
|
Limit of Bis(4-sulfobutyl) ether disodium /IC;USP<621>;USP<1065> |
NMT 0.05% |
|
Bacterial Endotoxins Test/USP<85> |
≤10EU/g |
|
Microbial Enumeration Tests/USP<61> |
TAMC≤100cfu/g TYMC≤50 cfu/g |
|
Tests for Specified Microorganisms/USP<62> |
Absence of Escherichia Coli/1g |
|
Clarity of Solution(30%,w/v)/visual;see details in the USP Monograph |
The solution is clear, and essentially free from particles of foreign matter. |
|
Average Degree of Substitution [DS]/CE;USP<1053> |
6.2-6.9 |
Each batch of Sulfobutyl Ether Beta Cyclodextrin Sodium (Injection Grade) is accompanied by a complete Certificate of Analysis (COA).
The COA covers appearance, identification, assay, impurity limits, bacterial endotoxins, microbial limits, and average degree of substitution in accordance with USP requirements.
COA and relevant technical documents are available upon request for quality review and regulatory reference.

1. Excellent water solubility suitable for injection formulations.
2. Forms stable inclusion complexes with a wide range of active pharmaceutical ingredients.
3. Improves solubility and bioavailability of poorly soluble drugs.
4. Reduces renal toxicity and minimizes hemolytic effects.
5. Helps control drug release rate and masks unpleasant odor or taste.
6. Injection grade quality complying with USP standards.
Xi'an DELI Biochemical Industry Co., Ltd. was established in 1999 and has focused on the research, development, and production of cyclodextrin and its derivatives for more than 26 years.
The company specializes in pharmaceutical excipients and supplies cyclodextrin-based products for pharmaceutical, veterinary, and chemical applications to global customers.
With stable production processes and strict quality management, Xi'an DELI Biochemical Industry Co., Ltd. is committed to providing reliable products and long-term cooperation.

Xi’an DELI Biochemical Industry Co., Ltd. has over 20 years of experience in the research, development, and production of cyclodextrin-based pharmaceutical excipients. The company focuses on providing high-quality, reliable, and compliant excipients to global pharmaceutical and formulation partners.
The production process is established with strict quality control at every stage, from raw material selection to finished product release. Each batch is manufactured under a well-defined quality management system and is accompanied by complete documentation, including Certificate of Analysis (COA) and traceability records.
The company’s sulfobutyl ether beta-cyclodextrin sodium is produced in accordance with USP requirements and is widely used in parenteral, oral, and other pharmaceutical formulations. Stable batch-to-batch consistency ensures reliable performance in drug solubilization, stabilization, and formulation development.
With a dedicated technical team, Xi’an DELI Biochemical Industry Co., Ltd. provides professional technical support, formulation guidance, and regulatory documentation assistance to meet customer-specific application and registration needs.

Q1: What is the main function of Sulfobutyl Ether Beta Cyclodextrin Sodium?
It is mainly used as a solubilizer and complexing agent to improve drug solubility, stability, and safety in pharmaceutical formulations.
Q2: Is this product suitable for injectable formulations?
Yes, it is injection grade and complies with USP requirements for parenteral use.
Q3: Does the product form covalent complexes with drugs?
No, it forms stable non-covalent inclusion complexes with drug molecules.
Q4: Can this excipient reduce drug toxicity?
Yes, it can help reduce renal toxicity and hemolytic effects compared with some traditional solubilizers.
Q5: What dosage forms can use this product?
It is suitable for injectable, oral, nasal, and ophthalmic dosage forms.
Q6: Is technical support available?
Yes, technical support and application guidance can be provided upon request.
Q7: What packaging sizes are available?
Standard packaging includes 500 g, 1 kg, and 10 kg. Customized packaging is available.
Q8: What is the shelf life of the product?
The shelf life is 36 months when stored in sealed and proper conditions.
 Betadex Sulfobutyl Ether Sodium CAS NO 182410-00-0
Betadex Sulfobutyl Ether Sodium CAS NO 182410-00-0 Sulfobutyl Ether Beta Cyclodextrin Sodium
Sulfobutyl Ether Beta Cyclodextrin Sodium Betadex Sulfobutyl Ether Sodium Injection Grade
Betadex Sulfobutyl Ether Sodium Injection Grade Betadex Sulfobutyl Ether Sodium SBECD Injectable Grade for Drug Formulations
Betadex Sulfobutyl Ether Sodium SBECD Injectable Grade for Drug Formulations Betadex Sulfobutyl Ether Sodium CAS 182410-00-0
Betadex Sulfobutyl Ether Sodium CAS 182410-00-0 DMF Betadex Sulfobutyl Ether Sodium 182410-00-0
DMF Betadex Sulfobutyl Ether Sodium 182410-00-0