
Betadex Sulfobutyl Ether Sodium Injectable Pharmaceutical Excipient EP USP Grade (CAS 182410-00-0) is a highly water-soluble cyclodextrin derivative used as a solubilizer and stabilizer in injectable drug formulations, enhancing solubility, safety, and bioavailability.
Xi'an Deli Biochemical Industry Co., Ltd. is a high-tech enterprise specializing in research, development, production, and sales of cyclodextrin derivatives. Established in 1999, the company has over 25 years of experience in pharmaceutical excipients, including Hydroxypropyl Betadex (HPBCD) and Betadex Sulfobutyl Ether Sodium (SBECD). The company operates dedicated clean areas and advanced production lines, ensuring high-quality, stable, and consistent products for global pharmaceutical applications.
Betadex Sulfobutyl Ether Sodium Injectable Pharmaceutical Excipient EP USP Grade (CAS 182410-00-0) is a highly water-soluble anionic cyclodextrin derivative. SBECD functions as a solubilizer, wetting agent, chelating agent, and masking agent, enhancing drug solubility, stability, and safety in injectable, oral, nasal, and ophthalmic formulations. SBECD is widely used in antifungal, antiviral, cardiovascular, and oncology APIs.

CAS No: 182410-00-0
Molecular Formula: C42H70-nO35·(C4H8SO3Na)n
Grade: Injectable, EP and USP compliant
Appearance: White to off-white amorphous powder
Assay: ≥99.0%
Applications: Injectable and parenteral pharmaceutical formulations
Packaging: 500g/bag; 1kg/bag; 10kg/bag; 10kg/drum; customized packaging available
Storage: Sealed and dry
Shelf Life: 36 months
COA / Quality Assurance:
Each batch of SBECD is tested for assay purity, bacterial endotoxin, residual solvents, heavy metals, and average degree of substitution (DS), complying with USP and EP pharmacopeial standards.
Betadex Sulfobutyl Ether Sodium is used to enhance solubility, reduce renal toxicity, minimize hemolysis, and improve patient compliance. SBECD is suitable for intravenous, intramuscular, nasal, oral, and ophthalmic delivery systems. It forms inclusion complexes with weakly basic and nitrogen-containing APIs, providing sustained release and improved stability.
Hydroxypropyl Betadex (HPBCD) – Injectable and oral pharmaceutical excipient
Betadex Sulfobutyl Ether Sodium (SBECD) – Injectable-grade cyclodextrin derivative 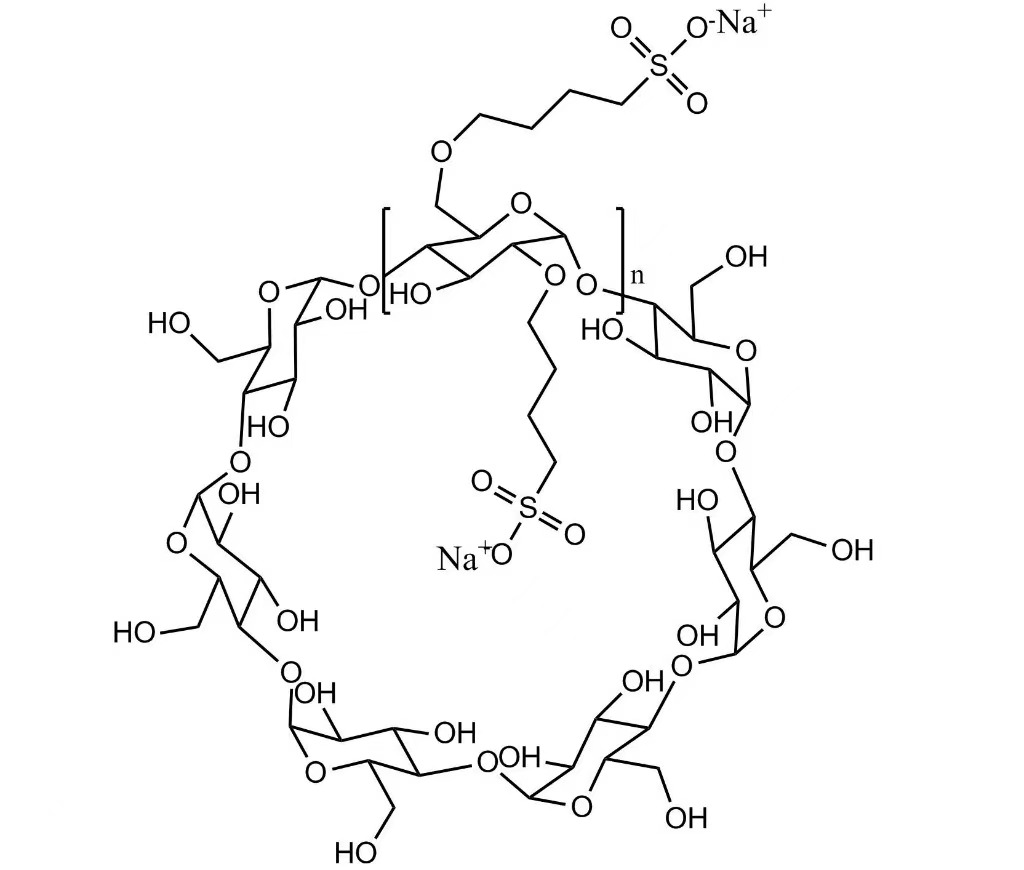
Q: Is Xi'an Deli Biochemical the manufacturer of SBECD?
A: Yes, Xi'an Deli Biochemical produces Betadex Sulfobutyl Ether Sodium (SBECD) and HPBCD. Beta Cyclodextrin is used as the raw material for derivative production.
Q: Are samples available?
A: Yes, SBECD samples are available for evaluation.
Q: What is the batch and annual production capacity?
A: SBECD batch production capacity is 2.5 tons, and annual output exceeds 200 tons.
Q: What regulatory documents are provided?
A: COA, MSDS, stability data, and technical dossiers are available for all batches.
Q: Shipping options?
A: Global shipping by air, sea, or express courier.


 Betadex Sulfobutyl Ether Sodium CAS NO 182410-00-0
Betadex Sulfobutyl Ether Sodium CAS NO 182410-00-0 Sulfobutyl Ether Beta Cyclodextrin Sodium
Sulfobutyl Ether Beta Cyclodextrin Sodium Betadex Sulfobutyl Ether Sodium Injection Grade
Betadex Sulfobutyl Ether Sodium Injection Grade Betadex Sulfobutyl Ether Sodium SBECD Injectable Grade for Drug Formulations
Betadex Sulfobutyl Ether Sodium SBECD Injectable Grade for Drug Formulations Betadex Sulfobutyl Ether Sodium CAS 182410-00-0
Betadex Sulfobutyl Ether Sodium CAS 182410-00-0 DMF Betadex Sulfobutyl Ether Sodium 182410-00-0
DMF Betadex Sulfobutyl Ether Sodium 182410-00-0