
Hydroxypropyl Betadex HPBCD for Pharmaceutical Applications. Consistent batches, 3MT+, 500MT/year capacity. Technical data and samples available on request.Hydroxypropyl Betadex CAS 128446-35-5 Reliable Excipient Since 1999
Hydroxypropyl Betadex Injection Grade (CAS 128446-35-5) is a highly water-soluble beta-cyclodextrin derivative used as a pharmaceutical excipient to improve the solubility, stability, and bioavailability of poorly water-soluble active pharmaceutical ingredients (APIs).
By introducing hydroxypropyl groups into the beta-cyclodextrin structure, this excipient shows significantly enhanced aqueous solubility and improved safety compared with native beta-cyclodextrin, making it suitable for oral and parenteral formulations.
Hydroxypropyl Betadex is a non-ionic cyclodextrin derivative with excellent inclusion complex formation capability. It functions as an effective solubilizer, stabilizer, and bioavailability enhancer in pharmaceutical formulations, helping to reduce or eliminate the use of organic solvents.
Through reversible non-covalent interactions, hydrophobic drug molecules are encapsulated within the cyclodextrin cavity while maintaining a hydrophilic exterior. This mechanism improves drug solubility and protects APIs from degradation without altering their chemical structure.
Hydroxypropyl Betadex is widely used in oral solid and liquid dosage forms to enhance dissolution rate, improve bioavailability, and provide taste masking for poorly soluble drugs.
It is also suitable for parenteral formulations following formulation evaluation, as well as ophthalmic, nasal, and topical products where high solubility, low irritation, and good safety profiles are required.
In addition, Hydroxypropyl Betadex is effective in chemical applications, such as stabilizing reactive compounds and controlling the release of active substances. Its water solubility and inclusion capability make it an essential additive for complex formulations.
We offer detailed technical data and documentation for evaluation, ensuring that customers can confidently use Hydroxypropyl Betadex in research and production. Whether for pharmaceutical development, cosmetic formulations, or chemical applications, this excipient delivers stable performance and reliable results.

Product Name: Hydroxypropyl Betadex
CAS No.: 128446-35-5
Molecular Formula: C42H70O35·(C3H6O)n
Grade: Pharmaceutical Grade / Injection Grade (optional)
Standard: USP / EP / Enterprise Standard
Application: Pharmaceutical excipient
Appearance: White or almost white powder
Purity: >99%
Packaging: 500 g/bag; 1 kg/bag; 10 kg/bag; 10 kg/drum (customized packaging available)
| Description & Solubility | White to off-white amorphous powder; freely soluble in water |
| Identification (IR / HPLC) | Complies with USP / EP |
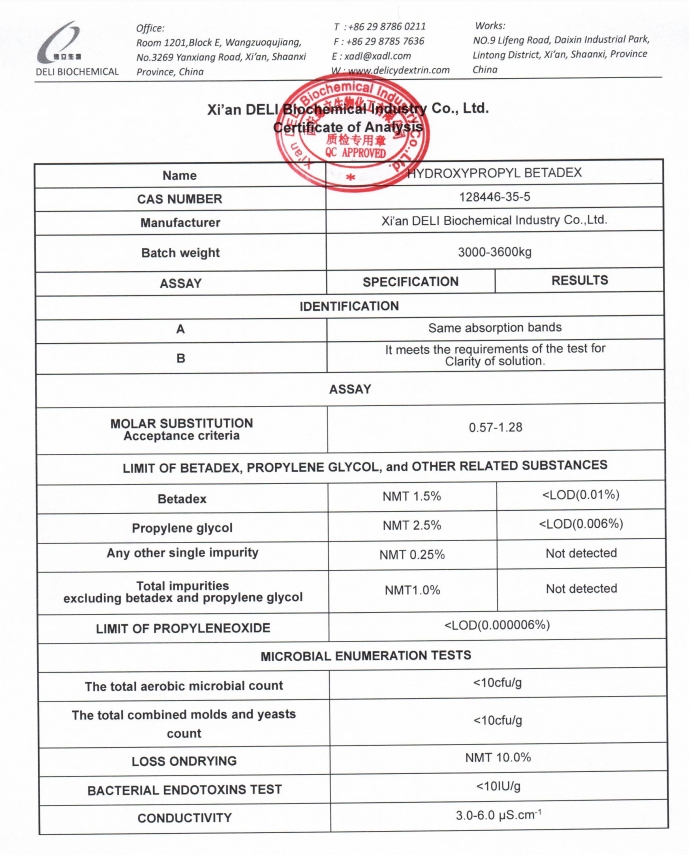
| Assay | 95.0% – 105.0% |
| Degree of Substitution | Controlled and consistent |
| Loss on Drying | ≤ 10.0% |
|
LIMIT OF PROPYLENEOXIDE |
NMT 0.0001% |
|
LIMIT OF BETADEX |
NMT 1.5% |
|
The total aerobic microbial count |
<10cfu/g |
|
The total combined molds and yeasts |
<10cfu/g |
Hydroxypropyl Betadex offers excellent water solubility and strong solubilization capability for poorly soluble APIs. Its non-ionic nature ensures good compatibility with a wide range of active pharmaceutical ingredients.
Compared with native beta-cyclodextrin, it demonstrates improved safety, reduced irritation risk, and reliable batch-to-batch consistency, supporting pharmaceutical development and commercial production.
The product is produced under a strict quality management system with full traceability from raw materials to finished product. Key attributes including purity, substitution degree, and microbial limits are closely monitored.
Complete technical documentation and regulatory support are available to facilitate formulation development and product registration in global markets.

Each batch of Hydroxypropyl Betadex is supplied with a detailed Certificate of Analysis (COA), including batch number, test methods, specification limits, and authorized quality approval.
Q: What is Hydroxypropyl Betadex used for?
A: It is used as a pharmaceutical excipient to improve solubility, stability, and bioavailability of poorly soluble APIs.
Q: Is it suitable for injectable formulations?
A: Injection grade material is available and suitable for parenteral use after formulation evaluation.
Q: Does it react chemically with APIs?
A: No. It forms reversible inclusion complexes without changing the API structure.
Q: Does the product comply with USP and EP standards?
A: Yes. It is manufactured in accordance with USP, EP, and enterprise quality standards.
Q: Is the product manufactured by your own factory?
A: Yes. Xi’an DELI is a direct manufacturer with in-house production, quality control, and technical support.
Xi’an DELI Biochemical Industry Co., Ltd. has extensive experience in the research, development, and manufacturing of cyclodextrin-based pharmaceutical excipients. With more than two decades of industry expertise, DELI focuses on delivering high-quality products that meet the stringent requirements of global pharmaceutical markets.
As a manufacturer with integrated production facilities, DELI ensures stable supply capacity and strict control over the entire manufacturing process, from raw material sourcing to finished product release. Controlled processes and validated methods help guarantee consistent batch-to-batch quality and reliable performance in pharmaceutical formulations.
With a strong commitment to quality, transparency, and customer-focused service, Xi’an DELI has established long-term partnerships with pharmaceutical and biotechnology companies worldwide and continues to be a trusted supplier of cyclodextrin excipients for injectable and other non-oral dosage forms.

