
Hydroxypropyl Βetadex CAS 128446-35-5
DMF 034772
Specification: 500g/Bag; 10kg/Bag, or as your requirement
Category: Pharmaceutical excipients
Storage: Keep airtight and in a dry place.
Period of validity: 36 months
Hydroxypropyl Betadex is a chemically modified beta-cyclodextrin widely used as a pharmaceutical excipient to enhance the solubility, stability, and bioavailability of poorly water-soluble active pharmaceutical ingredients (APIs). By introducing hydroxypropyl groups into the cyclodextrin molecule, this product achieves significantly improved aqueous solubility and reduced toxicity compared with native beta-cyclodextrin.
Due to its favorable safety profile and excellent inclusion complex capability, Hydroxypropyl Betadex is suitable for a wide range of pharmaceutical dosage forms, including oral and non-oral formulations, subject to formulation evaluation.
Hydroxypropyl Betadex is a non-ionic cyclodextrin derivative characterized by a hydrophobic inner cavity and a hydrophilic outer surface. This unique molecular structure enables it to form reversible inclusion complexes with hydrophobic drug molecules through non-covalent interactions.
By encapsulating APIs within its cavity, Hydroxypropyl Betadex improves drug solubility and dissolution rate, protects sensitive ingredients from hydrolysis, oxidation, and photodegradation, and enhances formulation stability without altering the chemical structure of the active ingredient.
Hydroxypropyl Betadex is widely applied in pharmaceutical formulations that require enhanced solubility and improved bioavailability. In oral solid and liquid dosage forms, it supports faster dissolution, taste masking, and improved formulation stability.
Following appropriate formulation assessment, it may also be used in parenteral, ophthalmic, nasal, and topical preparations where high solubility, low irritation, and good biocompatibility are required.
Product Name: Hydroxypropyl Betadex
CAS No.: 128446-35-5
Molecular Formula: C42H70O35·(C3H6O)n
Grade: Pharmaceutical Grade / Injection Grade (optional)
Appearance: White or almost white powder
Solubility: Freely soluble in water
Application: Pharmaceutical excipient
| Description | White to off-white amorphous powder; freely soluble in water |
| Identification | Complies with USP / EP requirements |
| Assay | 95.0% – 105.0% |
| Degree of Substitution | Controlled and consistent |
| Loss on Drying | ≤ 10.0% |
| Microbial Limits | Complies with pharmacopeial standards |
Hydroxypropyl Betadex provides excellent solubilization performance for poorly soluble APIs, allowing flexible formulation design and reducing reliance on organic solvents. Its non-ionic nature ensures broad compatibility with different classes of active ingredients.
Compared with native beta-cyclodextrin, this derivative demonstrates improved safety, lower irritation risk, and reduced precipitation tendency, supporting both development-stage and commercial pharmaceutical production.
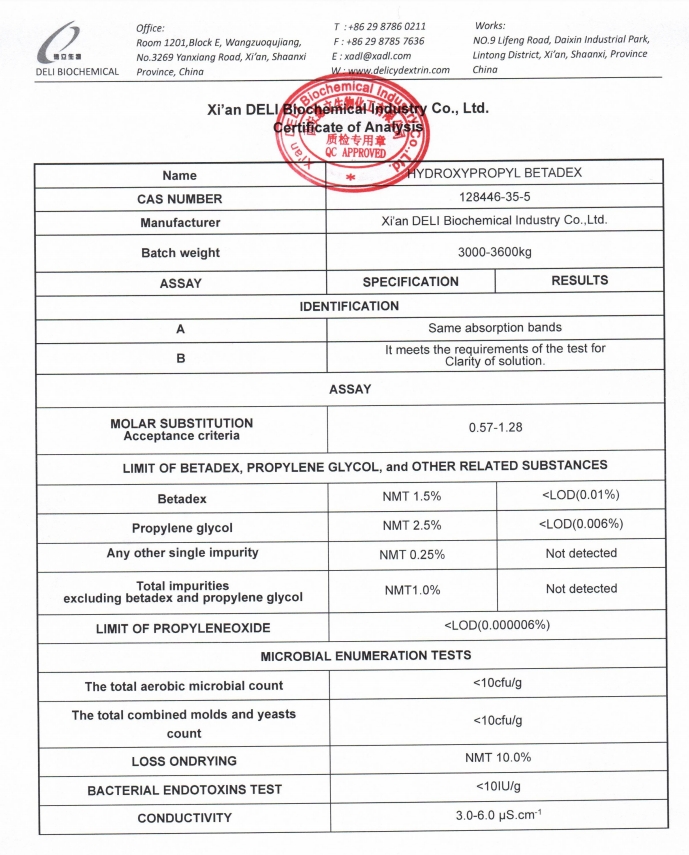
Hydroxypropyl Betadex is produced under a comprehensive quality management system with strict control over critical quality attributes. Each batch is supplied with a complete and traceable Certificate of Analysis (COA).
Supporting technical documentation and regulatory information are available to assist customers during formulation development and product registration.
Xi’an DELI Biochemical Industry Co., Ltd. has focused on the research, development, and production of cyclodextrin-based excipients for more than two decades. The company has accumulated extensive technical experience in beta-cyclodextrin derivatives, including Hydroxypropyl Betadex for pharmaceutical applications.
All key production processes are completed under an integrated and controlled manufacturing system, ensuring stable quality, consistent substitution degree, and reliable batch-to-batch performance. Strict in-process controls and validated analytical methods are applied throughout raw material handling, synthesis, purification, and final product release.
Xi’an DELI operates under a comprehensive quality management framework aligned with international pharmaceutical requirements. The company supports USP and EP compliance and provides complete documentation, including Certificate of Analysis, technical data, and regulatory support materials, to assist customers during formulation development and registration.
With stable production capacity and long-term supply capability, DELI is able to support both development-stage projects and commercial-scale demand. Flexible packaging options and batch size control allow efficient support for laboratory evaluation, pilot studies, and routine production.
In addition to product quality, DELI places strong emphasis on technical communication and customer support. The technical team works closely with customers to address solubility challenges, excipient selection, and formulation optimization, helping to improve development efficiency and reduce formulation risk.

Q: What is Hydroxypropyl Betadex mainly used for?
A: It is mainly used as a pharmaceutical excipient to improve the solubility, stability, and bioavailability of poorly water-soluble APIs.
Q: Is it suitable for injectable formulations?
A: Injection grade material is available and may be used for parenteral formulations following formulation evaluation.
Q: Does Hydroxypropyl Betadex chemically react with APIs?
A: No. It forms reversible inclusion complexes without changing the chemical structure of the active ingredient.
Q: Does the product comply with USP and EP standards?
A: Yes. It is manufactured in accordance with USP, EP, and internal quality standards.