
Sulfobutyl Ether Beta Cyclodextrin Powder Injectable Grade Pharmaceutical Excipient
Product Name: Betadex Sulfobutyl Ether Sodium
CAS No: 182410-00-0
Pharmacopeial Standard: EP and USP
Appearance: White to off-white amorphous powder
Assay: ≥99.0%
Applications: Injectable and parenteral pharmaceutical formulations
Packaging: 500 g/bag; 1 kg/bag; 10 kg/bag; 10 kg/drum; customized packaging available
Sulfobutyl Ether Beta Cyclodextrin Powder Injectable Grade Pharmaceutical Excipient (CAS 182410-00-0) is a highly water-soluble, anionic β-cyclodextrin derivative specifically developed for injectable pharmaceutical formulations. SBECD functions as a solubilizer, wetting agent, chelating agent, and masking agent, enhancing solubility, stability, and safety of poorly soluble APIs.
This injectable-grade excipient is suitable for intravenous, intramuscular, and subcutaneous drug delivery. SBECD reduces renal toxicity, minimizes hemolysis, stabilizes nitrogen-containing APIs, and enables controlled release while masking unpleasant drug odors.
Xi'an DELI Biochemical Industry Co., Ltd. was established in 1999 and has over 26 years of experience in the research, production, and commercialization of cyclodextrin derivatives. Core products include Sulfobutyl Ether Beta Cyclodextrin (SBECD) and Hydroxypropyl Betadex (HPBCD). DELI provides high-purity, consistent, and regulatory-compliant products for global pharmaceutical markets.
DELI operates advanced production lines and dedicated quality control systems to ensure batch-to-batch consistency. COA, technical documentation, and formulation support are available to assist R&D, clinical development, and commercial production worldwide.
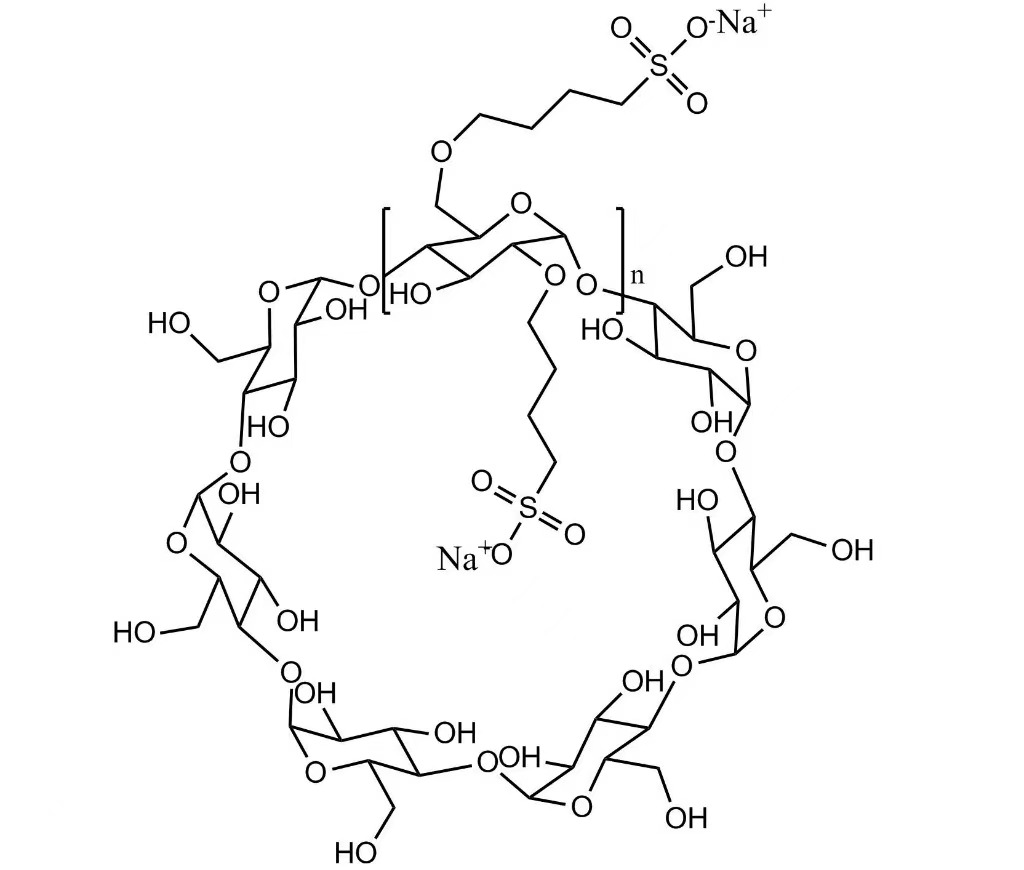
CAS No: 182410-00-0
Molecular Formula: C42H70-nO35·(C4H8SO3Na)n
Grade: Injectable, EP and USP compliant
Appearance: White to off-white amorphous powder
Assay: ≥99.0%
Applications: Injectable and parenteral pharmaceutical formulations
Packaging: 500g/bag; 1kg/bag; 10kg/bag; 10kg/drum; customized packaging available
Storage: Sealed and dry
Shelf Life: 36 months
COA / Quality Assurance: Each batch is tested for assay purity, bacterial endotoxins, residual solvents, heavy metals, and average degree of substitution (DS), meeting USP and EP standards.
SBECD is used to improve solubility, stability, and safety of injectable APIs. It is applied in antifungal, antiviral, cardiovascular, and oncology injectable drugs. SBECD forms inclusion complexes with nitrogen-containing drugs, providing controlled release and reducing toxicity.
Q: Is Xi'an DELI Biochemical the manufacturer of SBECD?
A: Yes, DELI produces Sulfobutyl Ether Beta Cyclodextrin (SBECD) and Hydroxypropyl Betadex (HPBCD). Beta Cyclodextrin is the raw material for derivatives.
Q: Are samples available?
A: Yes, samples of SBECD are available for evaluation.
Q: What is the batch and annual production capacity?
A: Batch production capacity is 2.5 tons; annual output exceeds 200 tons.
Q: What regulatory documents are provided?
A: COA, MSDS, stability data, and technical dossiers are available.
Q: Shipping options?
A: Global shipping via air, sea, or express courier.
Contact Xi'an DELI Biochemical to request samples, technical documentation, or quotations for Sulfobutyl Ether Beta Cyclodextrin Powder Injectable Grade Pharmaceutical Excipient. Our team supports formulation development with reliable supply and professional expertise.

 Betadex Sulfobutyl Ether Sodium CAS NO 182410-00-0
Betadex Sulfobutyl Ether Sodium CAS NO 182410-00-0 Sulfobutyl Ether Beta Cyclodextrin Sodium
Sulfobutyl Ether Beta Cyclodextrin Sodium Betadex Sulfobutyl Ether Sodium Injection Grade
Betadex Sulfobutyl Ether Sodium Injection Grade Betadex Sulfobutyl Ether Sodium SBECD Injectable Grade for Drug Formulations
Betadex Sulfobutyl Ether Sodium SBECD Injectable Grade for Drug Formulations Betadex Sulfobutyl Ether Sodium CAS 182410-00-0
Betadex Sulfobutyl Ether Sodium CAS 182410-00-0 DMF Betadex Sulfobutyl Ether Sodium 182410-00-0
DMF Betadex Sulfobutyl Ether Sodium 182410-00-0