
Betadex Sulfobutyl Ether Sodium powder for Injectable and Parenteral Formulations, SBECD is widely used in the development of modern drugs due to its unique ability to enhance the solubility and bioavailability of APIs. It is especially useful in the formulation of antiviral, antifungal, anticancer, and cardiovascular drugs.
Betadex Sulfobutyl Ether Sodium powder for Injectable and Parenteral Formulations
Betadex Sulfobutyl Ether Sodium (SBECD) is a highly purified, non-toxic, anionic cyclodextrin derivative specifically developed for parenteral drug delivery systems, including injectable, ophthalmic, and inhalation formulations.
It enhances the solubility, stability, and bioavailability of poorly water-soluble active pharmaceutical ingredients (APIs), without the toxicity risks associated with traditional solubilizers.
Manufactured by Xi’an Deli Biochemical Industry Co., Ltd., the product meets USP and EP standards and is supported by more than 26 years of expertise in cyclodextrin manufacturing.
SBECD is widely used in modern drug development due to its unique ability to improve API solubility and bioavailability, especially in injectable and parenteral formulations.
If you would like a quotation, COA, or trial order, please contact us:
Email: xadl@xadl.com
Company: Xi’an Deli Biochemical Industry Co., Ltd.
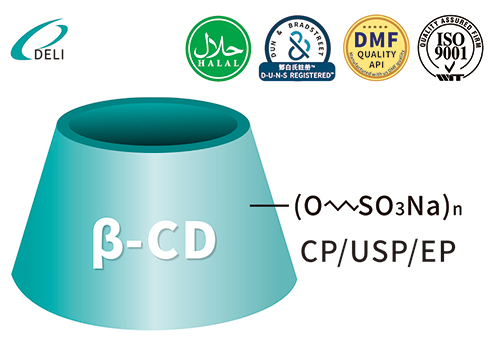 Betadex Sulfobutyl Ether Sodium CAS NO 182410-00-0
Betadex Sulfobutyl Ether Sodium CAS NO 182410-00-0 Sulfobutyl Ether Beta Cyclodextrin Sodium
Sulfobutyl Ether Beta Cyclodextrin Sodium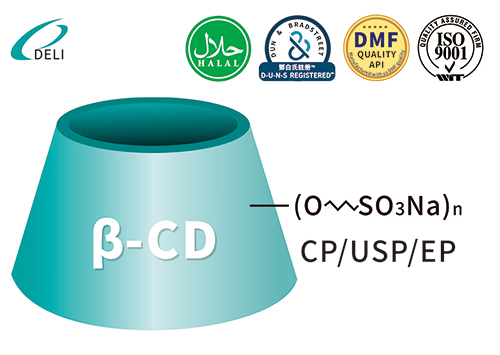 Betadex Sulfobutyl Ether Sodium Injection Grade
Betadex Sulfobutyl Ether Sodium Injection Grade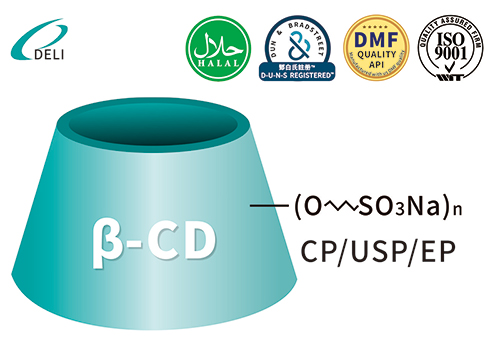 Betadex Sulfobutyl Ether Sodium SBECD Injectable Grade for Drug Formulations
Betadex Sulfobutyl Ether Sodium SBECD Injectable Grade for Drug Formulations Betadex Sulfobutyl Ether Sodium CAS 182410-00-0
Betadex Sulfobutyl Ether Sodium CAS 182410-00-0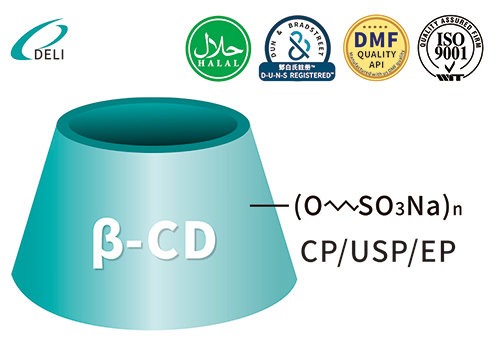 DMF Betadex Sulfobutyl Ether Sodium 182410-00-0
DMF Betadex Sulfobutyl Ether Sodium 182410-00-0