


Sulfobutyl Beta Cyclodextrin Sodium CAS 182410-00-0 is a highly water-soluble cyclodextrin derivative used as a pharmaceutical excipient. This injectable grade SBECD improves solubility, stability, and safety of poorly soluble APIs in parenteral and oral formulations.
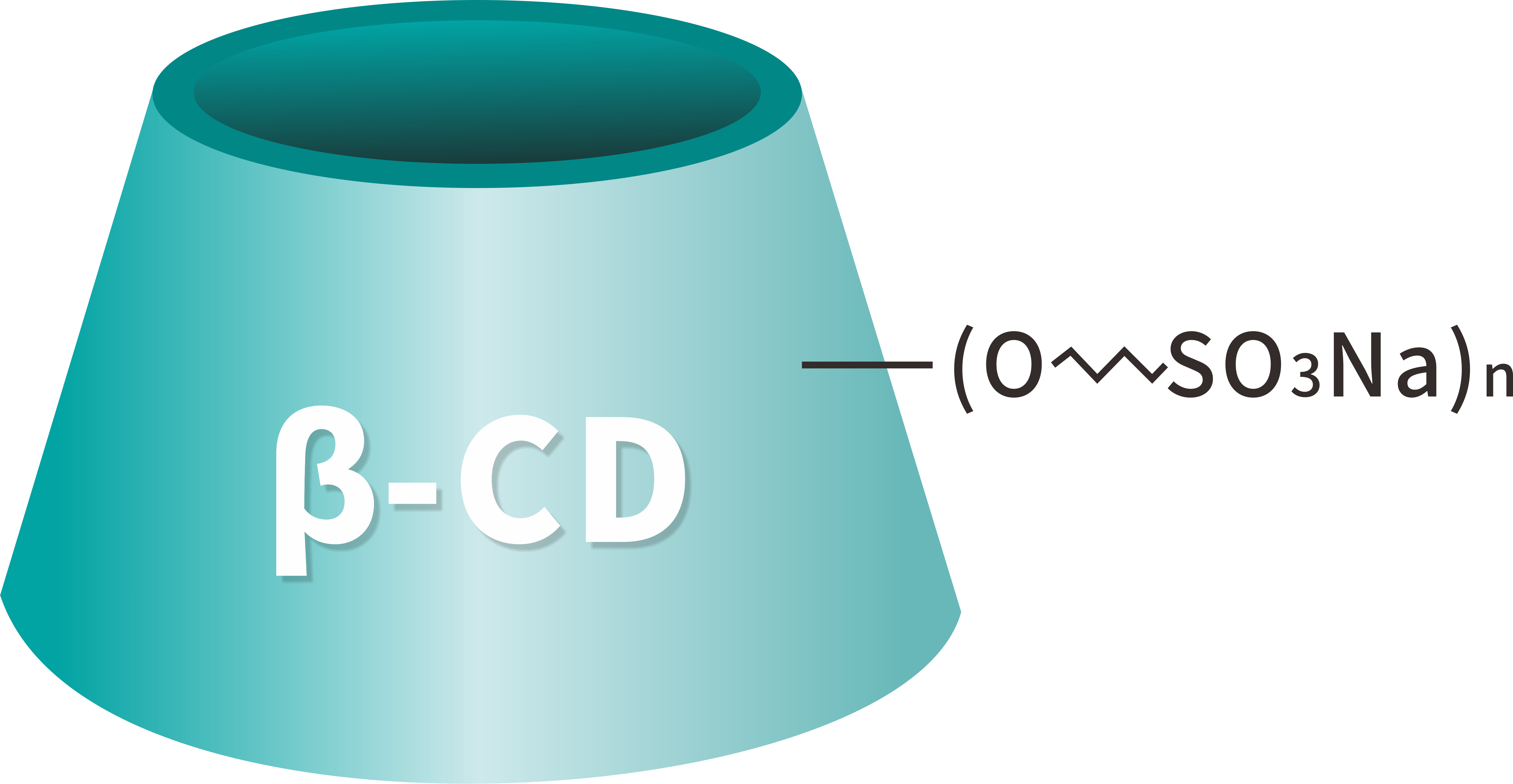
Sulfobutyl Beta Cyclodextrin Sodium CAS 182410-00-0, also known as Sulfobutyl Beta Cyclodextrin Sodium (SBECD), is a highly water-soluble anionic cyclodextrin derivative developed for pharmaceutical excipient applications. Betadex Sulfobutyl Ether Sodium CAS No. 182410-00-0 is widely used as a solubilizer, wetting agent, chelating agent (complexing agent), and polyvalent masking agent in advanced drug formulations.
Betadex Sulfobutyl Ether Sodium CAS No. 182410-00-0 has been successfully applied in injectable, oral, nasal, and ophthalmic drug formulations. Sulfobutyl Beta Cyclodextrin Sodium shows strong inclusion capability and specific affinity toward nitrogen-containing drugs, effectively improving drug solubility, stability, and formulation safety through non-covalent complex formation.
In pharmaceutical applications, Betadex Sulfobutyl Ether Sodium CAS No. 182410-00-0 helps reduce renal toxicity, minimize hemolysis, control drug release rates, and mask unpleasant odors, making Sulfobutyl Beta Cyclodextrin Sodium especially suitable for parenteral formulations requiring high safety and performance.
[CAS No.]: 182410-00-0
[Molecular Formula]: C42H70-nO35·(C4H8SO3Na)n
[Grade]: Injectable / Pharmaceutical Grade
[Executive Standard]: USP / EP / Enterprise Standard
[Specification]: 500 g/bag; 1 kg/bag; 10 kg/bag; 10 kg/drum
[Application Area]: Pharmaceutical excipients
| Description and Solubility | White to off-white amorphous powder. Freely soluble in water; sparingly soluble in methanol; practically insoluble in ethanol, n-hexane, 1-butanol, acetonitrile, 2-propanol, and ethyl acetate. |
| Identification A (IR), USP <197K> | Complies with SBECD reference standard |
| Identification B (HPLC), USP <621> | Retention time of major peak complies with SBECD reference |
| Identification C (CE), USP <1053> | Meets requirements for Average Degree of Substitution |
| Identification D, USP <191> | Positive reaction for sodium |
| Assay (HPLC), USP <621> | 95.0% – 105.0% (anhydrous basis) |
| Limit of Betadex | NMT 0.1% |
| Limit of 1,4-Butane Sultone | NMT 0.5 ppm |
| Limit of Sodium Chloride | NMT 0.2% |
| Bacterial Endotoxins, USP <85> | ≤10 EU/g |
| Microbial Limits, USP <61> |
TAMC ≤ 100 cfu/g TYMC ≤ 50 cfu/g |
| Specified Microorganisms, USP <62> | Absence of Escherichia coli in 1 g |
| Average Degree of Substitution (DS) | 6.2 – 6.9 |
The pharmaceutical potential of beta-cyclodextrin derivatives has been confirmed through decades of research. Compared with native beta-cyclodextrin, Betadex Sulfobutyl Ether Sodium CAS 182410-00-0 demonstrates improved safety and solubilization performance, making Sulfobutyl Beta Cyclodextrin Sodium an effective excipient for enhancing the bioavailability of poorly soluble drugs, especially BCS Class II compounds.
Xi’an Deli Biochemical Industry Co., Ltd. provides full technical support, including COA, MSDS, and formulation assistance. Sample evaluation and customized packaging solutions are available upon request.
For formulation development, regulatory documentation, or commercial supply inquiries related to Betadex Sulfobutyl Ether Sodium CAS No. 182410-00-0, please contact Xi’an Deli Biochemical Industry Co., Ltd. Our technical team supports projects from R&D to commercial scale.


 Betadex Sulfobutyl Ether Sodium CAS NO 182410-00-0
Betadex Sulfobutyl Ether Sodium CAS NO 182410-00-0 Sulfobutyl Ether Beta Cyclodextrin Sodium
Sulfobutyl Ether Beta Cyclodextrin Sodium Betadex Sulfobutyl Ether Sodium Injection Grade
Betadex Sulfobutyl Ether Sodium Injection Grade Betadex Sulfobutyl Ether Sodium SBECD Injectable Grade for Drug Formulations
Betadex Sulfobutyl Ether Sodium SBECD Injectable Grade for Drug Formulations Betadex Sulfobutyl Ether Sodium CAS 182410-00-0
Betadex Sulfobutyl Ether Sodium CAS 182410-00-0 DMF Betadex Sulfobutyl Ether Sodium 182410-00-0
DMF Betadex Sulfobutyl Ether Sodium 182410-00-0