
Keywords: Betadex Sulfobutyl Ether Sodium
Betadex Sulfobutyl Ether Sodium has revolutionised pharmaceutical excipient technology. This highly water-soluble cyclodextrin derivative enables the formation of stable inclusion complexes with active pharmaceutical ingredients (APIs), making it possible to formulate drugs that were previously limited by poor solubility and instability.
By significantly improving injectable drug solubility, bioavailability, and safety, Betadex Sulfobutyl Ether Sodium has become a critical component in modern drug delivery systems. Its unique molecular structure allows hydrophobic drug molecules to become clinically viable through efficient molecular encapsulation.
Poorly soluble compounds continue to challenge drug delivery systems. Traditional formulation strategies often fail to achieve adequate solubility or stability, preventing many promising drug candidates from reaching patients. Modern pharmaceutical research increasingly relies on advanced excipients to overcome these limitations while maintaining patient safety.
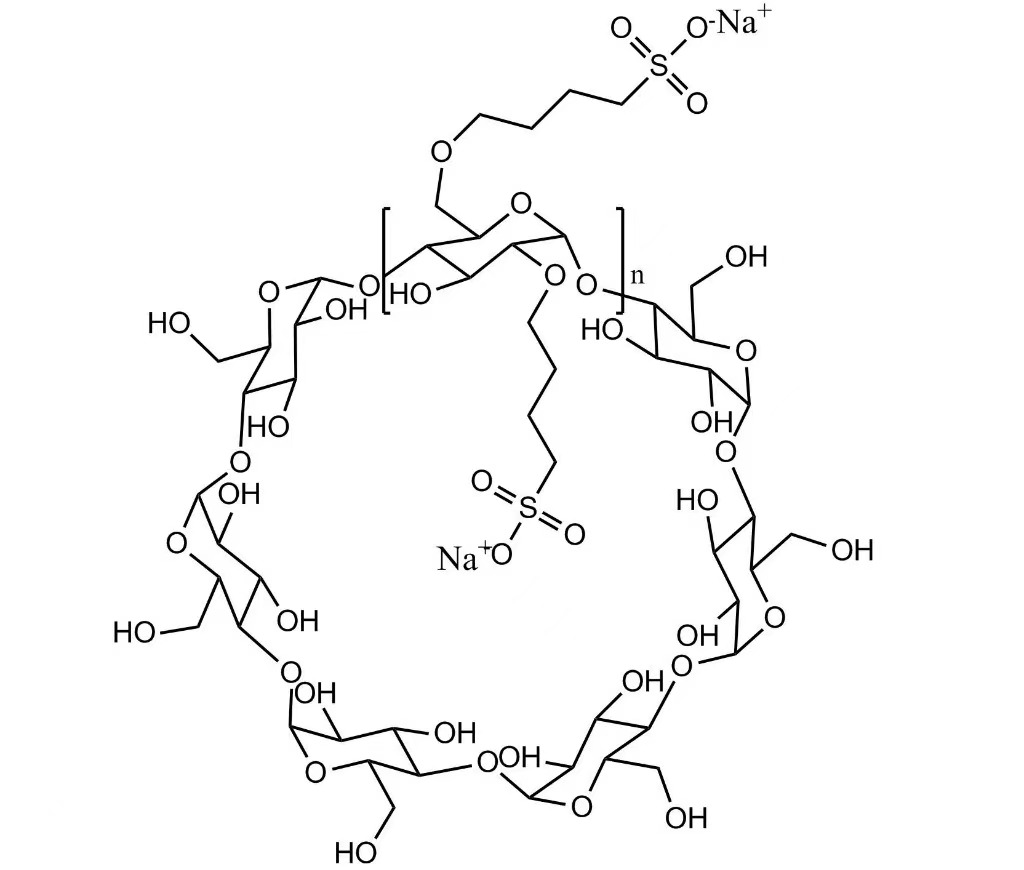
Sulfobutyl ether cyclodextrin is an anionic cyclodextrin derivative created by introducing sulfobutyl groups onto the beta-cyclodextrin backbone. This structural modification dramatically enhances aqueous solubility compared with native cyclodextrins.
The excipient forms non-covalent inclusion complexes with drug molecules. The hydrophobic inner cavity of the cyclodextrin hosts lipophilic APIs, while the hydrophilic outer surface maintains water solubility. This encapsulation improves pharmacokinetics and protects sensitive compounds from chemical degradation.
The degree of substitution directly influences solubilisation efficiency. Higher substitution levels improve solubility, but optimal performance requires balancing solubilising power with safety.
Scientific studies demonstrate that Betadex Sulfobutyl Ether Sodium exhibits lower haemolytic activity and reduced renal accumulation compared with many conventional solubilizers, making it suitable for repeated parenteral administration.
Many antifungal agents suffer from poor water solubility, limiting their injectable use. Betadex Sulfobutyl Ether Sodium forms stable complexes with drugs such as voriconazole and posaconazole, enabling effective injectable formulations.
Beyond solubility improvement, complexation enhances pH tolerance and storage stability, extending shelf life and improving therapeutic outcomes for invasive fungal infections.
Antiviral drugs often encounter solubility barriers that restrict parenteral delivery. Sulfobutyl ether cyclodextrin enables stable injectable formulations by protecting APIs from degradation and enabling controlled release.
The success of remdesivir formulations highlights the importance of cyclodextrin-based solubilization in treating severe viral infections where rapid and reliable drug delivery is critical.
Many anticancer agents exhibit low aqueous solubility, hindering clinical development. Betadex Sulfobutyl Ether Sodium allows formulation of these potent compounds while maintaining a favourable safety profile.
Its low tissue accumulation and reduced toxicity are particularly valuable for oncology therapies requiring repeated dosing, enabling higher therapeutic exposure with improved tolerability.
Emergency cardiovascular care often relies on injectable drugs with rapid onset. This excipient enables stable formulations of poorly soluble cardioactive agents.
The low haemolytic activity of Betadex Sulfobutyl Ether Sodium makes it especially suitable for patients with compromised cardiac function who cannot tolerate haemolytic stress.
Pediatric and geriatric patients frequently face challenges with oral dosage forms. Injectable and liquid formulations using sulfobutyl ether cyclodextrin provide reliable drug delivery across age groups.
Molecular encapsulation also masks unpleasant taste, improving patient compliance, particularly in paediatric formulations.
Rare disease therapies often involve APIs with complex physicochemical properties. Betadex Sulfobutyl Ether Sodium helps overcome solubility challenges without extensive excipient redevelopment.
Its established safety profile and scalable manufacturing reduce regulatory and development risks, accelerating orphan drug commercialization.
Cyclodextrin encapsulation protects APIs from hydrolysis, oxidation, and photodegradation by physically shielding reactive groups.
This stability enhancement supports global distribution, especially in regions with limited cold-chain infrastructure.
Xi'an Deli Biochemical Industry Co., Ltd. brings over 26 years of expertise in cyclodextrin manufacturing. Every batch is rigorously tested for substitution degree, impurities, and endotoxins.
With an annual production capacity exceeding 200 metric tonnes of Betadex Sulfobutyl Ether Sodium, our facilities ensure stable, long-term supply for both clinical and commercial applications.
Validated manufacturing processes minimise batch-to-batch variability, allowing pharmaceutical customers to design robust and reproducible formulations.

Our products comply with major pharmacopoeial requirements, providing pharmaceutical manufacturers with consistent quality and regulatory confidence across global markets.
Expanding scientific literature and regulatory approvals continue to support broader adoption of Betadex Sulfobutyl Ether Sodium in advanced drug delivery systems.
Optimising complex formation kinetics and thermodynamics is essential for achieving desired solubility enhancement and drug release profiles.
Inclusion complexes may require adapted analytical methods to accurately quantify APIs. Validated, specific analytical techniques are critical.
Compatibility studies ensure long-term formulation stability when multiple excipients are used in combination.
Gene therapy, cell therapy, and personalised medicine represent promising areas for cyclodextrin-based delivery technologies.
Cyclodextrin derivatives enable fixed-dose combinations with controlled release and improved stability.
Hybrid systems combining cyclodextrins with nanoparticles are opening new frontiers in targeted drug delivery.
As pharmaceutical development advances, Betadex Sulfobutyl Ether Sodium continues to address complex formulation challenges while maintaining an excellent safety profile. Its versatility across therapeutic areas makes it an essential excipient for next-generation drug delivery systems.
Q: What makes sulfobutyl ether cyclodextrin superior to other solubilizing agents?
A: It offers lower toxicity, reduced haemolysis, reversible complexation, and improved drug stability compared with organic solvents and surfactants.
Q: How does the degree of substitution affect performance?
A: Higher substitution improves solubility, but optimal performance requires balancing solubilisation efficiency and safety.
Q: Can it be used in oral formulations?
A: Yes, although primarily designed for injectables, it can enhance solubility in oral formulations when justified by therapeutic benefit.
Betadex Sulfobutyl Ether Sodium from Xi'an Deli Biochemical Industry Co., Ltd. provides a safe, stable, and scalable solution for advanced injectable drug formulations. Contact us at xadl@xadl.com to learn more.
