
Betadex Sulfobutyl Ether Sodium powder CAS 182410-00-0
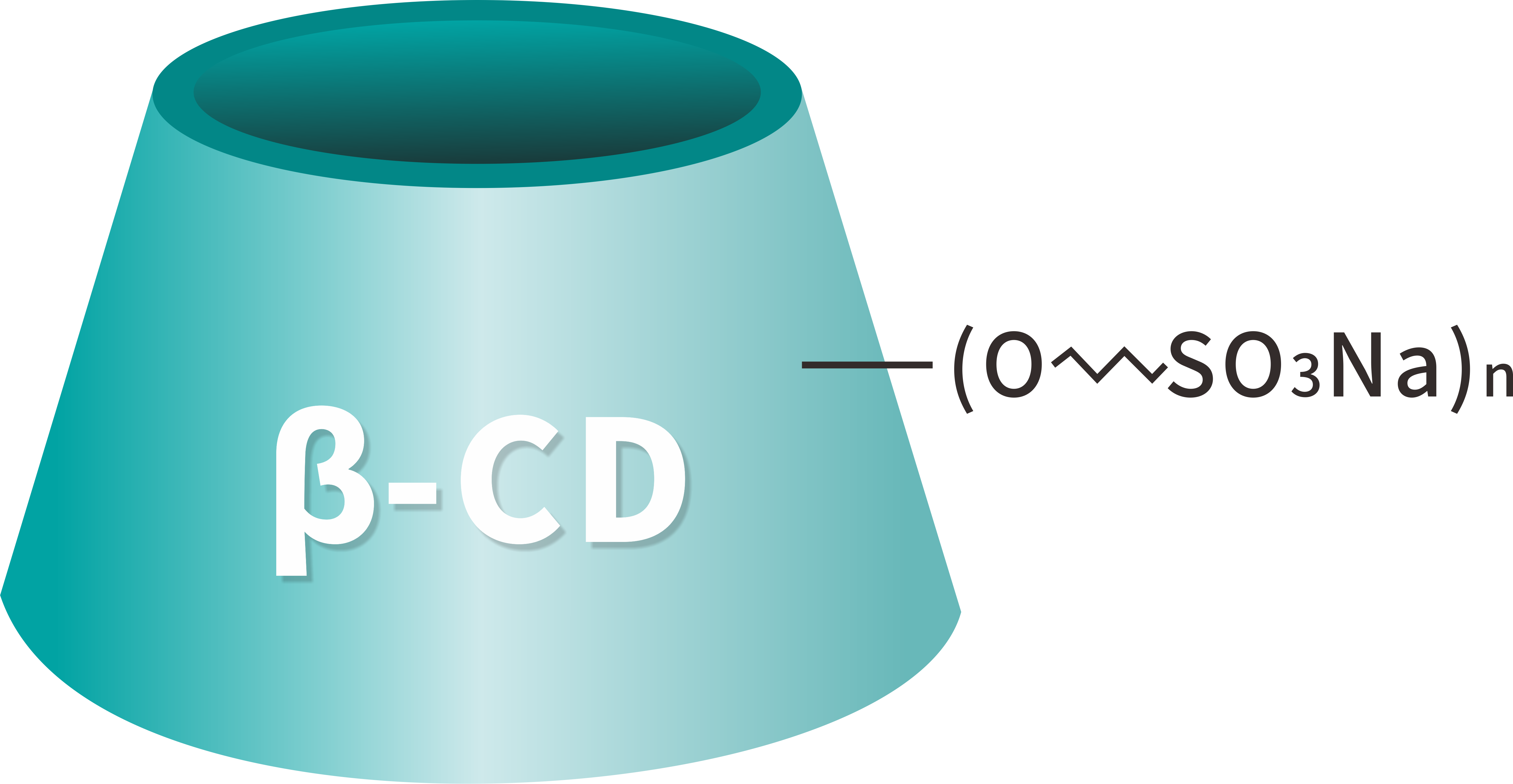
Molecular formula: C42H70-nO35•(C4H8SO3Na)n
Specification: 500g/Bag; 1kg/Bag; 10kg/bag/drum; or as your requirement
Category: Pharmaceutical excipients
Storage: Seal preservation.
Period of validity: 36 months
Betadex Sulfobutyl Ether Sodium Powder, also known as Sulfobutylether Beta Cyclodextrin Sodium (SBECD), is a highly water-soluble, anionic β-cyclodextrin derivative widely used as an injectable pharmaceutical excipient. With the CAS number 182410-00-0, this product is specifically designed to enhance the solubility, stability, and safety of poorly water-soluble active pharmaceutical ingredients (APIs).
Through the formation of stable, non-covalent inclusion complexes, Betadex Sulfobutyl Ether Sodium Powder enables the successful formulation of challenging APIs that are otherwise difficult to deliver via parenteral routes. Its excellent biocompatibility and low toxicity profile make it particularly suitable for repeated intravenous administration.
Betadex Sulfobutyl Ether Sodium Powder is produced by chemically modifying beta cyclodextrin with sulfobutyl ether groups under controlled alkaline conditions, followed by neutralization to form the sodium salt. This molecular modification significantly improves aqueous solubility compared to native beta cyclodextrin, while maintaining strong complexation capability with hydrophobic drug molecules.
The hydrophobic cavity of the cyclodextrin structure encapsulates lipophilic APIs, while the hydrophilic outer surface ensures excellent solubility in water. As a result, drug solubility, bioavailability, and formulation stability are greatly improved without the need for harsh organic solvents or surfactants.
Betadex Sulfobutyl Ether Sodium Powder is primarily used in injectable and parenteral drug formulations. It has been widely applied in antifungal, antiviral, oncology, and cardiovascular medicines, especially where high drug potency and poor water solubility present formulation challenges.
This excipient has been successfully used in marketed injectable products such as voriconazole, posaconazole, remdesivir, and other critical care medications, demonstrating its proven clinical performance and regulatory acceptance.
Product Name: Betadex Sulfobutyl Ether Sodium Powder
CAS No.: 182410-00-0
Appearance: White to off-white, amorphous, hygroscopic powder
Grade: Injectable pharmaceutical excipient
Standards: USP / EP / ChP
Assay: 95.0% – 105.0% (anhydrous basis)
Bacterial Endotoxins: ≤ 10 EU/g
Average Degree of Substitution: Controlled and consistent batch to batch

Xi’an Deli Biochemical Industry Co., Ltd. is a professional manufacturer specializing in cyclodextrin and cyclodextrin derivatives. Established in 1999, Deli Biochemical has over 25 years of dedicated experience in pharmaceutical excipient research, development, and industrial-scale production.
We focus on two core products: Hydroxypropyl Betadex (HPBCD) and Betadex Sulfobutyl Ether Sodium (SBECD). With long-term technical accumulation and continuous process optimization, our products are supplied to pharmaceutical customers worldwide for both clinical development and commercial manufacturing.
Our production facilities are designed to meet pharmaceutical manufacturing requirements, including controlled clean areas and strict process control. We operate large-scale production lines with an annual capacity of approximately 200 metric tons of Betadex Sulfobutyl Ether Sodium, ensuring stable supply for long-term cooperation.

Quality is at the core of our manufacturing philosophy. Each batch of Betadex Sulfobutyl Ether Sodium Powder undergoes comprehensive quality testing, including purity analysis, impurity profiling, endotoxin control, and degree of substitution verification.
Our quality management system is certified to ISO 9001 standards and supported by advanced analytical instruments such as HPLC, IC, GC, and capillary electrophoresis. Full Certificates of Analysis (COA) are provided with every shipment to ensure traceability and regulatory compliance.

With decades of experience in cyclodextrin manufacturing, Xi’an Deli Biochemical offers more than just a product. We provide reliable quality, stable supply, and professional technical support throughout the product lifecycle. Our experience with global regulatory requirements allows us to support customers in different markets with confidence.
As a direct manufacturer, we offer consistent batch quality, competitive supply capability, and long-term cooperation for pharmaceutical partners seeking a dependable injectable excipient supplier.

For samples, technical documentation, or commercial inquiries regarding Betadex Sulfobutyl Ether Sodium powder CAS 182410-00-0, please contact Xi’an Deli Biochemical Industry Co., Ltd. Our team will be pleased to support your formulation and supply needs.
 Betadex Sulfobutyl Ether Sodium CAS NO 182410-00-0
Betadex Sulfobutyl Ether Sodium CAS NO 182410-00-0 Sulfobutyl Ether Beta Cyclodextrin Sodium
Sulfobutyl Ether Beta Cyclodextrin Sodium Betadex Sulfobutyl Ether Sodium Injection Grade
Betadex Sulfobutyl Ether Sodium Injection Grade Betadex Sulfobutyl Ether Sodium SBECD Injectable Grade for Drug Formulations
Betadex Sulfobutyl Ether Sodium SBECD Injectable Grade for Drug Formulations Betadex Sulfobutyl Ether Sodium CAS 182410-00-0
Betadex Sulfobutyl Ether Sodium CAS 182410-00-0 DMF Betadex Sulfobutyl Ether Sodium 182410-00-0
DMF Betadex Sulfobutyl Ether Sodium 182410-00-0