
Grad: USP/EP
Assay: ≥99.0%
CAS No. : 182410-00-0
Appearance: White to off-white powder
Applications: Injectable and parenteral formulations
Packaging: 500 g/bag; 10 kg/drum or customized
Free sample available for evaluation
Betadex Sulfobutyl Ether Sodium SBECD High Purity Cyclodextrin Excipient
Betadex Sulfobutyl Ether Sodium SBECD High Purity Cyclodextrin Excipient
High Purity Betadex Sulfobutyl Ether Sodium (SBECD), Pharmaceutical Grade, CAS No. 182410-00-0, is a highly water-soluble derivative of beta-cyclodextrin widely used as a pharmaceutical excipient. Betadex Sulfobutyl Ether Sodium functions primarily as a solubilizer and stabilizer for poorly soluble active pharmaceutical ingredients (APIs).
With excellent safety, biocompatibility, and strong inclusion complex formation ability, SBECD is commonly used in injectable, oral, and ophthalmic drug formulations.
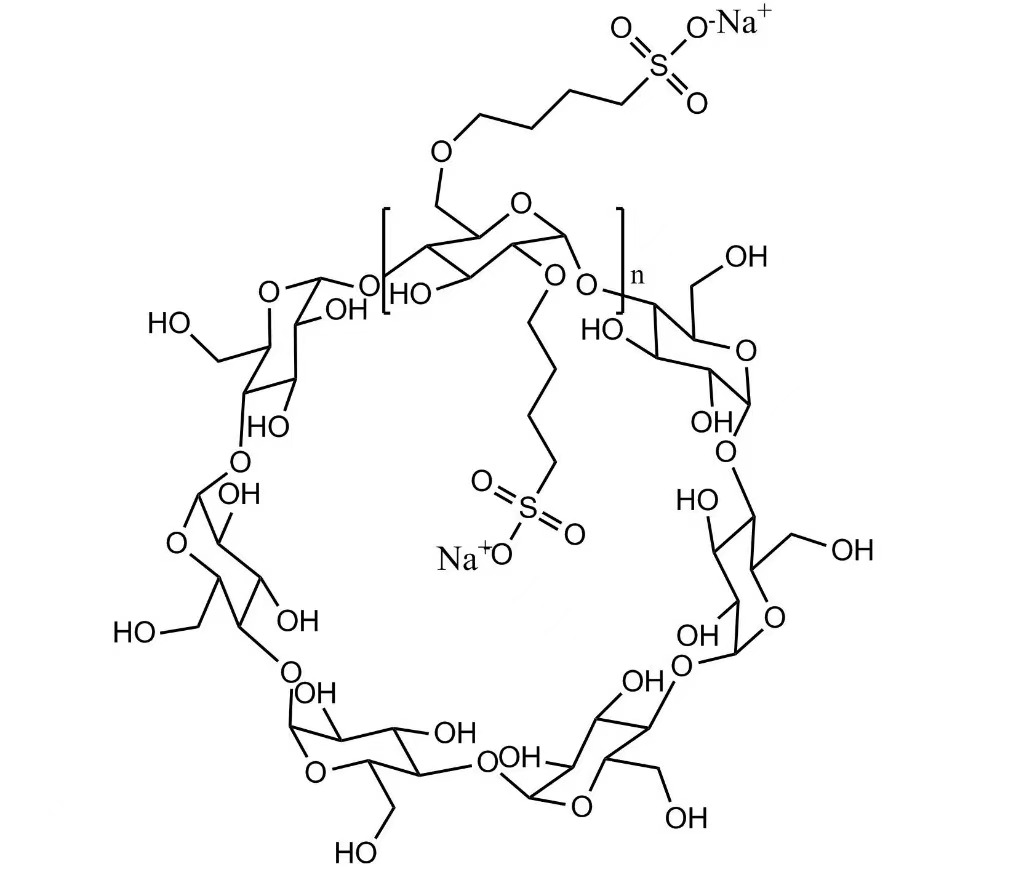
Betadex Sulfobutyl Ether Sodium is widely used in antifungal, antiviral, cardiovascular, and oncology drugs. It is an ideal choice for parenteral formulations where solubility enhancement and reduction of irritation are critical.
Our commercial products include Hydroxypropyl Betadex (HPBCD) and Betadex Sulfobutyl Ether Sodium (SBECD), exported globally and applied in various pharmaceutical preparations.
Xi’an Deli Biochemical Industry Co., Ltd. is a high-tech enterprise specializing in research, development, production, and sales of cyclodextrin derivatives since 1999. With over 26 years of experience, DELI provides stable supply and quality-assured HPBCD and SBECD to pharmaceutical companies worldwide.
Q1: Is DELI the manufacturer of SBECD?
A1: Xi’an Deli Biochemical Industry Co., Ltd. is the manufacturer of cyclodextrin derivatives such as Hydroxypropyl Betadex (HPBCD) and Betadex Sulfobutyl Ether Sodium (SBECD). Beta Cyclodextrin is used as the raw material in the production process.
Q2: What is the MOQ for SBECD?
A2: MOQ is 500g; free samples of 100g are available for evaluation.
Q3: What is the lead time?
A3: Typical lead time is 5–7 working days for trial orders. Production-scale orders are scheduled according to quantity.
For quotations, technical information, or sample requests of Betadex Sulfobutyl Ether Sodium SBECD, please contact DELI at xadl@xadl.com.


 Betadex Sulfobutyl Ether Sodium CAS NO 182410-00-0
Betadex Sulfobutyl Ether Sodium CAS NO 182410-00-0 Sulfobutyl Ether Beta Cyclodextrin Sodium
Sulfobutyl Ether Beta Cyclodextrin Sodium Betadex Sulfobutyl Ether Sodium Injection Grade
Betadex Sulfobutyl Ether Sodium Injection Grade Betadex Sulfobutyl Ether Sodium SBECD Injectable Grade for Drug Formulations
Betadex Sulfobutyl Ether Sodium SBECD Injectable Grade for Drug Formulations Betadex Sulfobutyl Ether Sodium CAS 182410-00-0
Betadex Sulfobutyl Ether Sodium CAS 182410-00-0 DMF Betadex Sulfobutyl Ether Sodium 182410-00-0
DMF Betadex Sulfobutyl Ether Sodium 182410-00-0