
CAS 182410-00-0 Sulfobutylether Beta Cyclodextrin Sodium is a cyclodextrin derivativea and a China DELI's new kind of pharmaceutical excipients.
Sulfobutylether Beta Cyclodextrin Sodium (SBECD) is a highly water-soluble, anionic β-cyclodextrin derivative developed as a pharmaceutical excipient for injectable and parenteral drug delivery systems. Through non-covalent inclusion complex formation with active pharmaceutical ingredients (APIs), SBECD significantly improves the solubility, stability, and formulation safety of poorly water-soluble drugs.
With excellent biocompatibility, low hemolytic activity, and controlled toxicity, Sulfobutylether Beta Cyclodextrin Sodium has become a widely accepted excipient in modern pharmaceutical formulations, especially in antiviral, antifungal, oncology, and cardiovascular therapies.
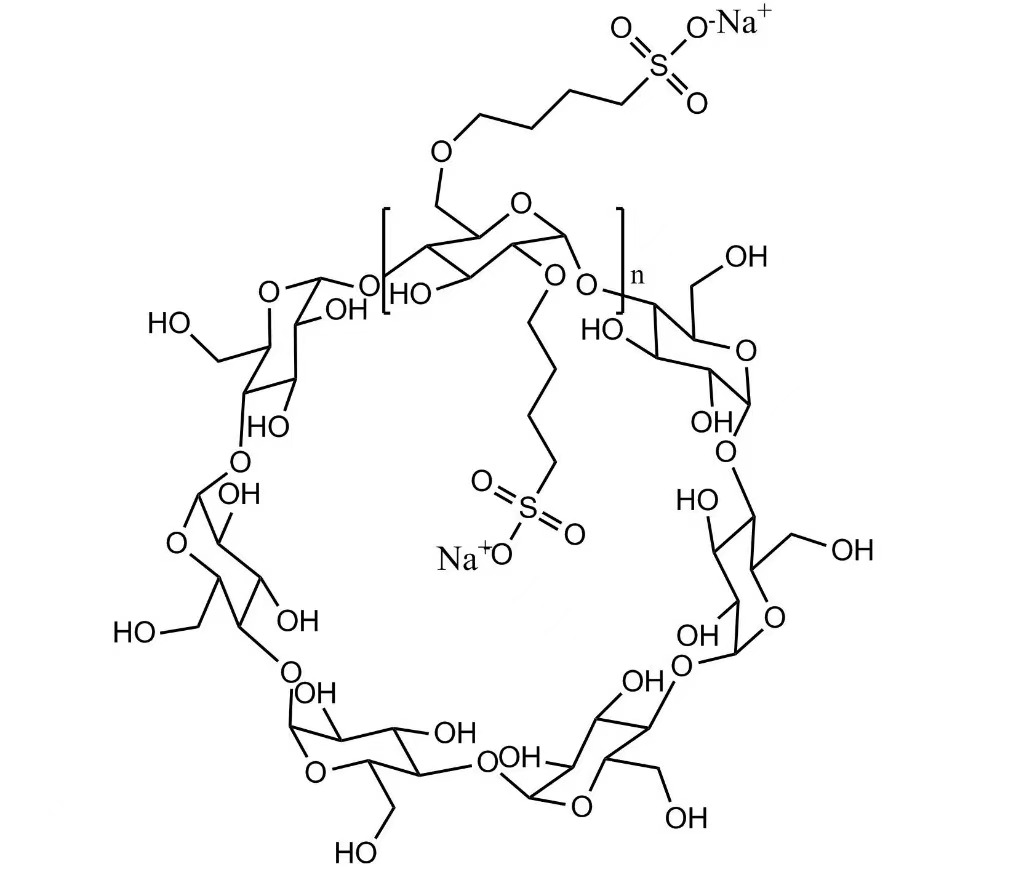
Sulfobutylether Beta Cyclodextrin Sodium appears as a white to off-white amorphous powder and is freely soluble in water. The product is supplied as injectable grade and manufactured in compliance with USP, EP, and relevant enterprise standards, ensuring suitability for regulated pharmaceutical applications.
Molecular Formula: C42H70-nO35·(C4H8SO3Na)n
Grade: Injection grade
Application Area: Pharmaceutical formulations
SBECD is an anionic derivative of betadex, in which sulfobutyl ether groups are introduced to the β-cyclodextrin structure. This chemical modification greatly enhances water solubility and enables the formation of stable yet reversible inclusion complexes with drug molecules.
Functionally, Sulfobutylether Beta Cyclodextrin Sodium acts as a solubilizer, stabilizer, and drug release modulator, improving formulation performance without chemically altering the active ingredient.

In pharmaceutical formulations, SBECD significantly enhances the aqueous solubility of poorly soluble APIs and improves both chemical and physical stability. Compared with conventional solubilizing agents, it exhibits reduced renal toxicity, minimal hemolytic potential, and low injection-site irritation.
SBECD also supports consistent bioavailability, controlled drug release, and, when applicable, effective masking of unpleasant odor or taste of APIs.
Sulfobutylether Beta Cyclodextrin Sodium is primarily used in parenteral dosage forms, including intravenous, intramuscular, and subcutaneous injections. It is also suitable for ophthalmic solutions, nasal formulations, and specialized drug delivery systems requiring enhanced solubility and stability.
The product is widely applied in antiviral, antifungal, oncology, cardiovascular, and central nervous system drug formulations.
SBECD has been successfully applied in several commercially marketed injectable products, demonstrating its proven safety and performance. Representative applications include remdesivir injection, voriconazole injection, posaconazole injection, melphalan hydrochloride for injection, and ziprasidone mesylate injection.
The injectable-grade product meets strict quality control requirements. Assay values are controlled between 95.0% and 105.0% on an anhydrous basis, with an average degree of substitution typically ranging from 6.2 to 6.9. Impurities, residual betadex, and inorganic salts are strictly limited.
Bacterial endotoxin levels comply with parenteral use requirements, and microbial limits conform to USP standards. Solution clarity and impurity profiles are monitored according to pharmacopeial methods. Each batch is supplied with a complete Certificate of Analysis (COA).
Xi’an Deli Biochemical Industry Co., Ltd. is a high-tech enterprise specializing in the research, development, production, and sales of cyclodextrins and their derivatives. Established in 1999, the company has more than 26 years of experience in pharmaceutical excipient manufacturing.
The company operates a modern production facility located in Lintong District, Xi’an, covering approximately 17.8 mu. Its core products include DELI brand Hydroxypropyl Betadex and Betadex Sulfobutyl Ether Sodium, both of which have been registered and filed with the FDA.
With an annual production capacity of 500 metric tons of Hydroxypropyl Betadex and 200 metric tons of Sulfobutylether Beta Cyclodextrin Sodium, Xi’an Deli Biochemical provides reliable, large-scale supply to pharmaceutical customers worldwide.

Xi’an Deli Biochemical operates under an ISO 9001 certified quality management system and is equipped with advanced analytical instruments such as HPLC, GC, ion chromatography, capillary electrophoresis, and FTIR. Strict quality control is applied from raw materials to finished products, ensuring excellent batch-to-batch consistency.

The product is available in 500 g, 1 kg, and 10 kg packaging options. Sulfobutylether Beta Cyclodextrin Sodium should be stored in a cool, dry place in tightly sealed containers and protected from moisture. Under recommended storage conditions, the shelf life is up to 36 months.
With decades of cyclodextrin manufacturing expertise, dedicated injectable-grade production lines, and proven global supply performance, Xi’an Deli Biochemical is a trusted partner for high-quality Sulfobutylether Beta Cyclodextrin Sodium. The company offers consistent quality, scalable production capacity, and strong technical support for formulation development.
For samples, technical data, or quotations for CAS 182410-00-0 Sulfobutylether Beta Cyclodextrin Sodium, please contact Xi’an Deli Biochemical Industry Co., Ltd.

 Betadex Sulfobutyl Ether Sodium CAS NO 182410-00-0
Betadex Sulfobutyl Ether Sodium CAS NO 182410-00-0 Sulfobutyl Ether Beta Cyclodextrin Sodium
Sulfobutyl Ether Beta Cyclodextrin Sodium Betadex Sulfobutyl Ether Sodium Injection Grade
Betadex Sulfobutyl Ether Sodium Injection Grade Betadex Sulfobutyl Ether Sodium SBECD Injectable Grade for Drug Formulations
Betadex Sulfobutyl Ether Sodium SBECD Injectable Grade for Drug Formulations Betadex Sulfobutyl Ether Sodium CAS 182410-00-0
Betadex Sulfobutyl Ether Sodium CAS 182410-00-0 DMF Betadex Sulfobutyl Ether Sodium 182410-00-0
DMF Betadex Sulfobutyl Ether Sodium 182410-00-0