
Another name for Betadex Sulfobutyl Ether Sodium is Sulfobutyl Ether Beta Cyclodextrin Sodium a new type of highly dissolved cyclodextrin, and Deli is a professional cyclodextrin manufacturer in China. Sulfobutyl Ether Beta Cyclodextrin Sodium Cas 182410-00-0
Injection Grade Betadex Sulfobutyl Ether Sodium CAS 182410-00-0 is a high-performance anionic beta-cyclodextrin derivative manufactured by Xi’an DELI Biochemical Industry Co., Ltd. It is developed as a pharmaceutical excipient to enhance the solubility, stability, and safety of poorly water-soluble active pharmaceutical ingredients.
The product features excellent aqueous solubility and a controlled degree of substitution, allowing it to form stable and reversible non-covalent inclusion complexes with a wide range of drug molecules. It is especially suitable for parenteral and other non-oral dosage forms that require strict quality and safety profiles.
Sulfobutyl Ether Beta Cyclodextrin Sodium CAS 182410-00-0 is a new generation cyclodextrin derivative with strong solubilizing and complexing capabilities. It can function as a solubilizer, wetting agent, chelating agent, and polyvalent masking agent in pharmaceutical formulations, helping to reduce the use of organic solvents and improve formulation tolerability.
The product shows special inclusion affinity for nitrogen-containing drugs and other poorly soluble APIs. By encapsulating drug molecules through non-covalent interactions, it improves drug solubility, stability, and bioavailability while reducing renal toxicity and hemolytic risk. In addition, it can help control drug release behavior and effectively mask unpleasant odor or taste.
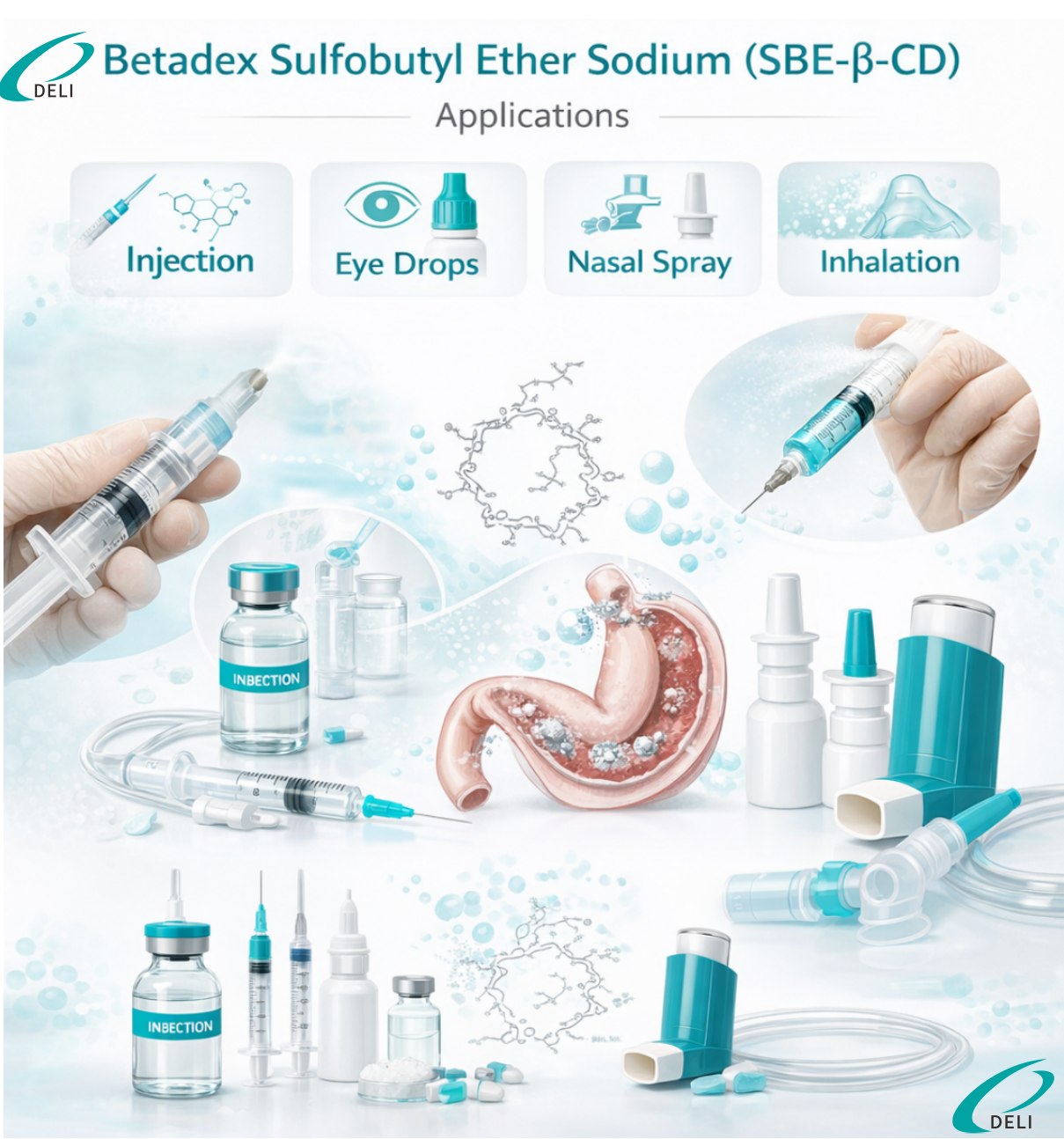
Injection Grade Betadex Sulfobutyl Ether Sodium is mainly applied in non-oral pharmaceutical dosage forms where high solubility and safety are critical. It is widely used in injectable solutions to improve aqueous solubility and maintain formulation clarity and stability during storage and administration.
In lyophilized powders for injection, the product helps protect sensitive APIs during freeze-drying and reconstitution processes, ensuring rapid dissolution and consistent performance. It is also suitable for ophthalmic formulations, nasal preparations, and inhalation systems following formulation evaluation, where low toxicity and good tolerability are required.
Product Name: Betadex Sulfobutyl Ether Sodium
CAS No.: 182410-00-0
Molecular Formula: C42H70-nO35·(C4H8SO3Na)n
Grade: Injection Grade
Executive Standard: USP / EP / Enterprise Standard
Application Area: Pharmaceutical Use
Packaging: 500 g/bag; 1 kg/bag; 10 kg/bag; 10 kg/drum; customized packaging available
| Description and Solubility | White to off-white amorphous powder. Freely soluble in water; sparingly soluble in methanol; practically insoluble in ethanol and common organic solvents. |
| Identification (IR / HPLC / CE) | Complies with USP requirements |
| Assay (HPLC) | 95.0% – 105.0% on anhydrous basis |
| Average Degree of Substitution | 6.2 – 6.9 |
| Residual Beta Cyclodextrin | NMT 0.1% |
| Bacterial Endotoxins | ≤ 10 EU/g |
| Microbial Limits | TAMC ≤ 100 cfu/g; TYMC ≤ 50 cfu/g |
The product provides excellent water solubility and strong solubilization capacity for poorly soluble APIs, making it suitable for complex injectable formulations. Through stable and reversible inclusion complex formation, it enhances formulation stability without altering the chemical structure of the drug substance.
Compared with native beta-cyclodextrin, it demonstrates lower toxicity, reduced hemolytic activity, and improved renal safety, supporting its application in parenteral and other sensitive dosage forms. Controlled substitution and high purity ensure reliable batch-to-batch consistency and predictable formulation performance.
The product is manufactured under a comprehensive quality management system with strict control from raw materials to finished product release. Key parameters such as impurity profile, endotoxin level, microbial limits, and degree of substitution are closely monitored to meet pharmaceutical standards.
Xi’an DELI Biochemical Industry Co., Ltd. holds complete company qualifications, including business license, production license, HALAL certification, and DMF support capability. Technical documents and regulatory-related materials can be provided upon request to support formulation development and product registration.
Each batch of Injection Grade Betadex Sulfobutyl Ether Sodium is released only after comprehensive quality testing and is supplied with a complete and traceable Certificate of Analysis (COA).
Typical COA test items include appearance, identification, assay, impurity profile, residual solvents where applicable, bacterial endotoxins, microbial limits, clarity of solution, and average degree of substitution. All test methods follow validated pharmacopeial or internal procedures.
Each COA includes product name, batch number, manufacturing date, retest date, test methods, specification limits, actual results, and authorized quality approval, ensuring full traceability from raw materials to finished product.
COA samples can be provided for quality evaluation, supplier qualification, and regulatory review upon request.

Q: What is the primary function of Injection Grade Betadex Sulfobutyl Ether Sodium?
A: It is mainly used as a pharmaceutical excipient to improve the solubility, stability, and safety of poorly water-soluble active pharmaceutical ingredients through reversible non-covalent inclusion complex formation.
Q: Is this product suitable for injectable formulations?
A: Yes. This product is injection grade and manufactured in accordance with USP and EP requirements, making it suitable for parenteral formulations after appropriate formulation evaluation.
Q: Does Betadex Sulfobutyl Ether Sodium chemically react with APIs?
A: No. It forms reversible non-covalent inclusion complexes without changing the chemical structure of the active pharmaceutical ingredient.
Q: What types of dosage forms can use this product?
A: It is mainly used in non-oral dosage forms, including injectable solutions, lyophilized powders for injection, ophthalmic formulations, nasal preparations, and inhalation systems, subject to formulation assessment.
Q: How does this product compare with native beta-cyclodextrin?
A: Compared with native beta-cyclodextrin, it offers significantly higher water solubility, lower toxicity, reduced hemolytic risk, and better suitability for parenteral applications.
Q: Is this product suitable for nitrogen-containing drugs?
A: Yes. It has strong inclusion affinity for nitrogen-containing compounds and is widely used to improve the solubility and stability of such APIs.
Q: What quality and regulatory documents are available?
A: Supporting technical documents, quality-related documentation, and regulatory materials such as DMF support information can be provided upon request.
Q: What packaging options are available?
A: Standard packaging includes 500 g, 1 kg, and 10 kg bags or drums. Customized packaging options are available according to customer requirements.
Q: What is the typical shelf life of the product?
A: The typical shelf life is 36 months when stored in sealed packaging under recommended storage conditions.
Q: Can technical support be provided during formulation development?
A: Yes. Xi’an DELI provides professional technical support and formulation guidance to assist customers throughout development and scale-up.
Xi’an DELI Biochemical Industry Co., Ltd. has over 20 years of experience in the research, development, and manufacturing of cyclodextrin-based pharmaceutical excipients. The company focuses on stable supply, consistent quality, and long-term cooperation with global pharmaceutical partners.
With professional technical support and reliable production capacity, DELI helps customers reduce formulation risks, improve development efficiency, and achieve sustainable commercial success.

 Betadex Sulfobutyl Ether Sodium CAS NO 182410-00-0
Betadex Sulfobutyl Ether Sodium CAS NO 182410-00-0 Sulfobutyl Ether Beta Cyclodextrin Sodium
Sulfobutyl Ether Beta Cyclodextrin Sodium Betadex Sulfobutyl Ether Sodium Injection Grade
Betadex Sulfobutyl Ether Sodium Injection Grade Betadex Sulfobutyl Ether Sodium SBECD Injectable Grade for Drug Formulations
Betadex Sulfobutyl Ether Sodium SBECD Injectable Grade for Drug Formulations Betadex Sulfobutyl Ether Sodium CAS 182410-00-0
Betadex Sulfobutyl Ether Sodium CAS 182410-00-0 DMF Betadex Sulfobutyl Ether Sodium 182410-00-0
DMF Betadex Sulfobutyl Ether Sodium 182410-00-0