
Wholesale Sulfobutylether Beta Cyclodextrin Sodium Salt CAS No. 182410-00-0 made in China from DELI. Our factory is a famous Sulfobutylether Beta Cyclodextrin Sodium Salt CAS No. 182410-00-0 manufacturers and suppliers in China. We will provide free sample. Our products are in stock. Welcome to buy our bulk products.
Xi'an Deli Biochemical Industry Co.,Ltd. Is a high-tech enterprise specializing in research & development, production & sales of cyclodextrin and its derivatives. Since its establishment on August 27, 1999,the company has been adhering to the quality Policy of "focusing on accessories, quality first, sincere service, striving for first-class". After more than 20 years of hard work and development, the company currently has products DELI Brand Hydroxypropyl Betadex, DELI Brand Betadex Sulfobutyl Ether Sodium. The above products have been registered and filed with the FDA.
Wholesale Sulfobutylether-β-Cyclodextrin sodium salt CAS 182410-00-0 made in China from DELI. Our factory is a famous Sulfobutylether-β-Cyclodextrin sodium salt CAS 182410-00-0 manufacturers and suppliers in China. We will provide free sample. Our products are in stock. Welcome to buy our bulk products.
Sulfobutylether-β-Cyclodextrin sodium salt CAS 182410-00-0 has been used in injection, oral, nasal and eye medicines. It has a special affinity and inclusion for nitrogen drugs. Increase the drug's stability ,solubility ,safety (Include drug molecules to form non-covalent). Reduce renal toxicity, ease drug hemolysis. Sulfobutyl Ether Beta Cyclodextrin Sodium also can control the drug release rate, cover the bad smell.
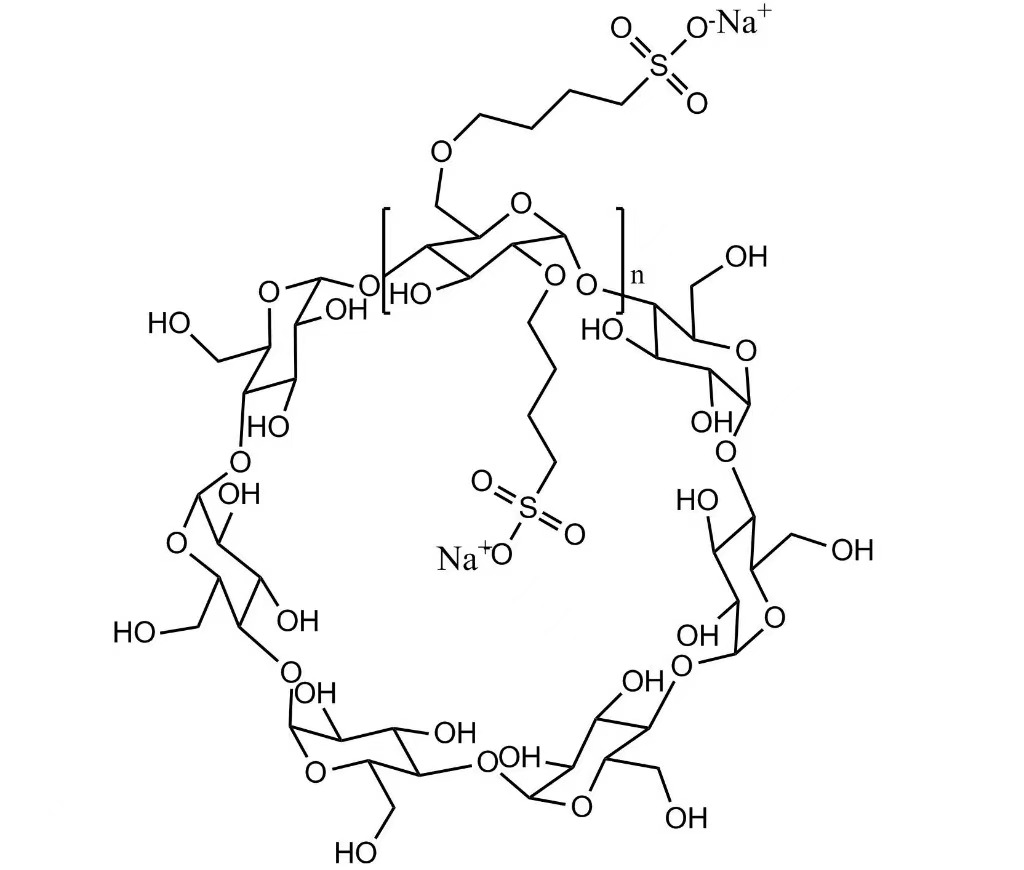
Sulfobutylether Beta Cyclodextrin Sodium Salt (SBECD) is a highly water-soluble, anionic β-cyclodextrin derivative developed as a pharmaceutical excipient for injectable drug delivery systems. By forming non-covalent inclusion complexes with active pharmaceutical ingredients (APIs), SBECD significantly improves solubility, stability, and formulation safety of poorly water-soluble drugs.
With excellent biocompatibility, low hemolytic potential, and controlled toxicity, SBECD is widely used in parenteral formulations for critical therapeutic areas including antiviral, antifungal, oncology, and cardiovascular medicines.
|
Description and Solubility/ see details in the USP Monograph |
White to off-white,amorphous powder.Freely soluble in water;sparingly soluble in methanol;practically insoluble in ethanol,in n-hexane,in 1-butanol,in acetonitrile,in 2-propanol,and in ethylacetate |
|
Identification A/IR; USP<197K> |
Complies with SBECD reference |
|
Identification B/(Assay method)HPLC; USP<621> |
tR of the major peak complies with SBECD reference |
|
Identification C/CE; USP<1053> |
It meets the requirements of the test for Average Degree of Substitution. |
|
Identification D/USP<191> |
Positive test for sodium |
|
Assay/HPLC;USP<621> |
95.0%-105.0% on the anhydrous basis |
|
Limit of Beta Cyclodextrin(Betadex)/IC; USP<621>;USP<1065> |
NMT 0.1% |
|
Limit of 1,4-Butane Sultone /GC;USP<621>, |
NMT 0.5ppm |
|
Limit of Sodium chloride /IC; USP<621>;USP<1065> |
NMT 0.2% |
|
Limit of 4-Hydroxybutane-1-sulfonic acid /IC;USP<621>;USP<1065> |
NMT 0.09% |
|
Limit of Bis(4-sulfobutyl) ether disodium /IC;USP<621>;USP<1065> |
NMT 0.05% |
|
Bacterial Endotoxins Test/USP<85> |
≤10EU/g |
|
Microbial Enumeration Tests/USP<61> |
TAMC≤100cfu/g TYMC≤50 cfu/g |
|
Tests for Specified Microorganisms/USP<62> |
Absence of Escherichia Coli/1g |
|
Clarity of Solution(30%,w/v)/visual;see details in the USP Monograph |
The solution is clear, and essentially free from particles of foreign matter. |
|
Average Degree of Substitution [DS]/CE;USP<1053> |
6.2-6.9 |
Sulfobutylether Beta Cyclodextrin Sodium Salt is primarily used in injectable and parenteral dosage forms, including:
Main therapeutic areas: antifungal, antiviral, oncology, cardiovascular medicines.
SBECD has been successfully applied in several commercially marketed injectable products, demonstrating its proven clinical performance:
A Certificate of Analysis (COA) is provided with each batch.

Xi’an Deli Biochemical Industry Co., Ltd. is an established manufacturer of injectable-grade SBECD with reliable commercial-scale supply capabilities.

For samples, technical data, or quotations for Sulfobutylether Beta Cyclodextrin Sodium Salt (CAS 182410-00-0), please contact Xi’an Deli Biochemical Industry Co., Ltd.
 Betadex Sulfobutyl Ether Sodium CAS NO 182410-00-0
Betadex Sulfobutyl Ether Sodium CAS NO 182410-00-0 Sulfobutyl Ether Beta Cyclodextrin Sodium
Sulfobutyl Ether Beta Cyclodextrin Sodium Betadex Sulfobutyl Ether Sodium Injection Grade
Betadex Sulfobutyl Ether Sodium Injection Grade Betadex Sulfobutyl Ether Sodium SBECD Injectable Grade for Drug Formulations
Betadex Sulfobutyl Ether Sodium SBECD Injectable Grade for Drug Formulations Betadex Sulfobutyl Ether Sodium CAS 182410-00-0
Betadex Sulfobutyl Ether Sodium CAS 182410-00-0 DMF Betadex Sulfobutyl Ether Sodium 182410-00-0
DMF Betadex Sulfobutyl Ether Sodium 182410-00-0