
USP Betadex Sulfobutyl Ether Sodium – the excipient solution to boost solubility, stability, and bioavailability of your drug formulations.
CAS No.: 182410-00-0
Category: Pharmaceutical Excipient
Function: Solubilizer/Complexing agent/Bioavailability enhancer
Solubility: Freely soluble in water
Application: Injectable formulations; Poorly soluble APIs; Drug stabilization and odor masking.
MOQ: 1 kg
OEM/ODM: Available
What is Betadex Sulfobutyl Ether Sodium?
USP Betadex Sulfobutyl Ether Sodium (SBECD) is a cyclodextrin derivative, a high-purity, water-soluble excipient specially designed for injectable and parenteral pharmaceutical formulations. It enhances the solubility, stability, and bioavailability of poorly water-soluble active pharmaceutical ingredients (APIs) by forming stable inclusion complexes, ensuring reliable drug delivery and consistent therapeutic performance.
SBECD is suitable for intravenous, intramuscular, and subcutaneous formulations, preventing precipitation, reducing chemical degradation, and maintaining the stability of sensitive APIs. It is also ideal for ophthalmic solutions, nasal sprays, and other liquid formulations requiring enhanced solubility and stability. Its high water solubility, low toxicity, and excellent biocompatibility make it a preferred excipient for research, clinical development, and commercial injectable products.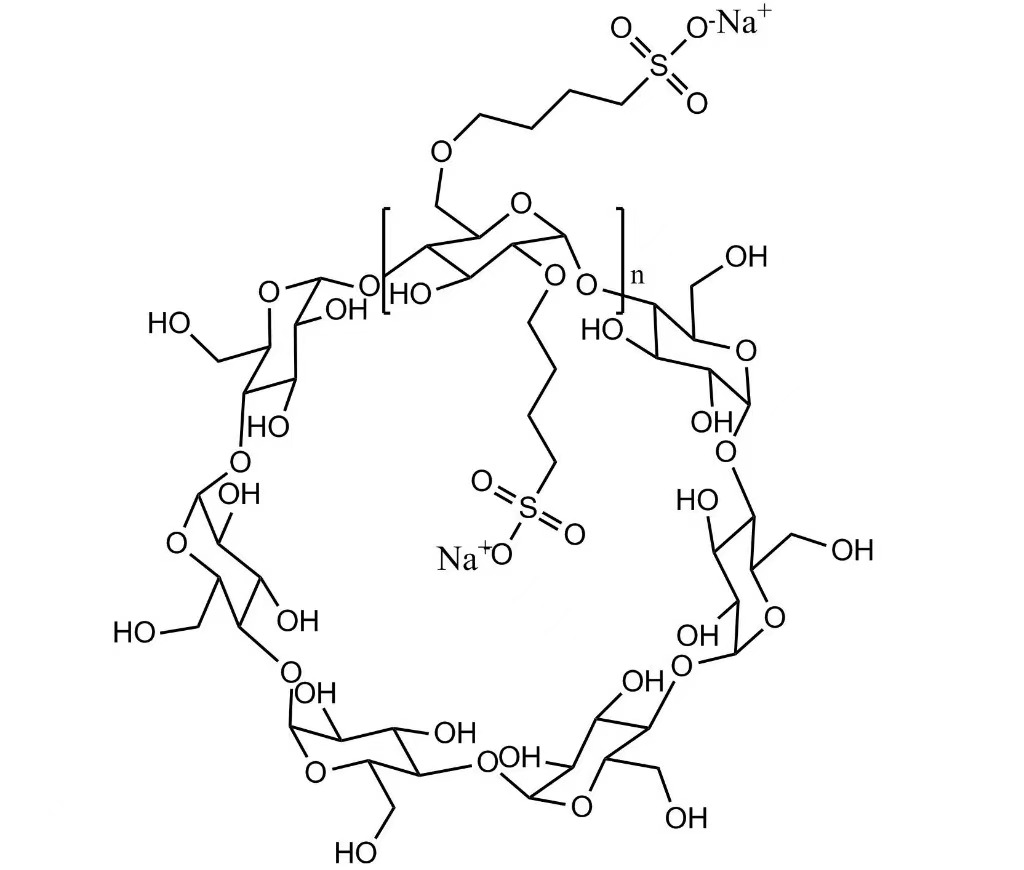
Applications and Typical Uses
Applications:
USP Betadex Sulfobutyl Ether Sodium (SBECD) is widely used as a pharmaceutical excipient to improve the solubility, stability, and bioavailability of poorly water-soluble drugs. It is suitable for injectable formulations, including intravenous, intramuscular, and subcutaneous injections, as well as ophthalmic solutions, nasal sprays, and other liquid formulations. SBECD can prevent precipitation, reduce chemical degradation, and enhance the stability of sensitive APIs, making it ideal for R&D, clinical development, and commercial pharmaceutical products.
Typical Drug Products Using SBECD:
Betadex Sulfobutyl Ether Sodium SBECD 182410-00-0 Specification
Product name
Betadex Sulfobutyl Ether Sodium
Other name
Sulfobutyl ether β-cyclodextrin sodium salt
Specification
USP/EP/JP/ChP
Category
Pharmaceutical Excipient
Shelf Time
36 months
MOQ
1 kg
Delivery Time
3-5 working days
Package
500g/bag; 1kg/bag; 10kg/fiber drum
Betadex Sulfobutyl Ether Sodium SBECD COA

Why Choose Us?
Xi’an Deli Biochemical Industry Co., Ltd. was established in 1999 and is a professional manufacturer specializing in cyclodextrin pharmaceutical excipients. With more than 25 years of continuous development, we have built solid technical expertise and a stable production system focused on high-quality cyclodextrin derivatives.
Our core products include Hydroxypropyl Betadex (HPβCD) and Betadex Sulfobutyl Ether Sodium (SBE-β-CD), which are widely used in pharmaceutical, veterinary, and chemical formulations for solubilization, stabilization, odor masking, and bioavailability enhancement of poorly soluble active ingredients. We ensure consistent batch-to-batch purity, reliable performance, and technical support for global pharmaceutical, veterinary, and cosmetic markets. Detailed technical data, certificates of analysis (COA), and sample evaluation are available to facilitate formulation development and optimize product performance.
• Over 25 years of manufacturing experience in cyclodextrin derivatives
• Stable production capacity with batch sizes of 2–3 metric tons
• Annual output up to 200 metric tons, ensuring reliable supply
• Focused products: Hydroxypropyl Betadex and Betadex Sulfobutyl Ether Sodium
• USP / EP / JP / ChP compliant, suitable for global pharmaceutical markets
• Consistent quality with strict batch-to-batch control
• Flexible supply, MOQ from 1 kg, supporting R&D to commercial production
We are committed to providing stable quality, reliable supply, and professional support for our global partners.

R&D & Technical Strength
Xi’an Deli Biochemical Industry Co., Ltd. has an in-house R&D team and complete production system, operating under a self-manufacturing and direct sales model. Our team focuses on excipient innovation and formulation support to meet diverse customer needs.
All production staff receive GMP training, and our laboratory is equipped with advanced instruments such as HPLC, GC, HPCE, IR, polarimeter, pH meter, conductivity meter, and moisture analyzer, ensuring reliable testing and consistent product quality.
The Quality Department works closely with Materials and Production to audit suppliers, retain samples, and conduct stability studies, maintaining high-quality excipients from R&D to market release.
Below you can see images of our team at international exhibitions and our certification documents, highlighting our technical strength and industry recognition.


FAQ
1. Do you have factory?
Yes, we are the factory with 26 years experience, welcome to visit our factory. When you have the details schedule, please kindly let me know in advance.
2. Do you have ISO and DMF?
Yes, we have obtained ISO9001, DMF, Halal Certificate and so on.
3. What is your delivery time?
As usual, our delivery time is about 1~3 working days after receipt of payments, however, for some special products, please kindly confirm with your sales manager in advance.
4. How can you confirm your quality?
Each batch of the goods can be shipped to you after strict inspection, if you still doubt, we can arrange the Pre-shipment Samples to your testing or to your pointed third party for retesting in advance, successfully, then we will arrange the bulk goods to you immediately.
5. What is your production capacity?
Our batch production capacity is 2.5 tons, and the annual output is 200 tons.
 Betadex Sulfobutyl Ether Sodium CAS NO 182410-00-0
Betadex Sulfobutyl Ether Sodium CAS NO 182410-00-0 Sulfobutyl Ether Beta Cyclodextrin Sodium
Sulfobutyl Ether Beta Cyclodextrin Sodium Betadex Sulfobutyl Ether Sodium Injection Grade
Betadex Sulfobutyl Ether Sodium Injection Grade Betadex Sulfobutyl Ether Sodium SBECD Injectable Grade for Drug Formulations
Betadex Sulfobutyl Ether Sodium SBECD Injectable Grade for Drug Formulations Betadex Sulfobutyl Ether Sodium CAS 182410-00-0
Betadex Sulfobutyl Ether Sodium CAS 182410-00-0 DMF Betadex Sulfobutyl Ether Sodium 182410-00-0
DMF Betadex Sulfobutyl Ether Sodium 182410-00-0