
Betadex Sulfobutyl Ether Sodium for Pharmaceutical Formulation
CAS No.: 182410-00-0
Appearance: White or nearly white hygroscopic powder
Executive Standard USP / EP / ChP
Packaging: 500 g/bag; 1 kg/bag; 10 kg/bag; 10 kg/drum
Synonyms: SBE-β-CD; Sulfobutyl Ether Beta-Cyclodextrin; Betadex SBE Sodium
Assay: ≥99%
Bacterial Endotoxins: ≤10 EU/g
MOQ: 1 kg
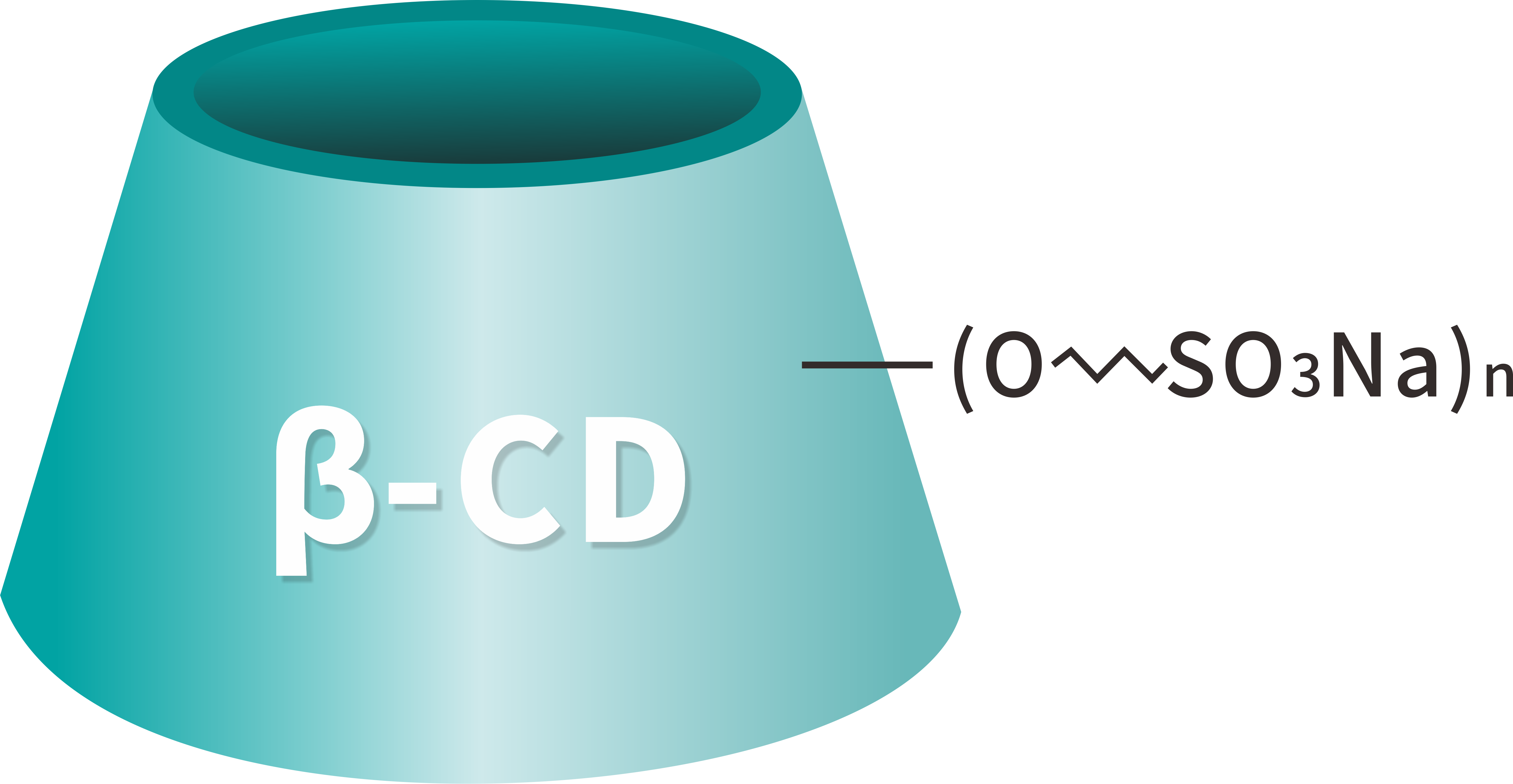
Betadex Sulfobutyl Ether Sodium is a highly water-soluble, anionic β-cyclodextrin derivative designed as a pharmaceutical excipient. It is synthesized by introducing sulfobutyl ether groups to beta-cyclodextrin under controlled alkaline conditions, forming a sodium salt with excellent solubilizing and complexation properties.
This injectable-grade excipient is intended to form stable, non-covalent inclusion complexes with poorly water-soluble active pharmaceutical ingredients (APIs). By encapsulating APIs, Betadex Sulfobutyl Ether Sodium enhances drug solubility, stability, and bioavailability while reducing hemolysis, renal toxicity, and local irritation at the injection site.
Thanks to its high safety profile and strong complexation ability, Betadex Sulfobutyl Ether Sodium is widely used in parenteral pharmaceutical formulations, including antifungal, antiviral, oncology, and cardiovascular injectable drugs. It meets major pharmacopeial standards such as USP and EP, and is suitable for both clinical and commercial pharmaceutical applications.
| Product Name | Betadex Sulfobutyl Ether Sodium |
| CAS No. | 182410-00-0 |
| Appearance | White or nearly white hygroscopic powder |
| Executive Standard | USP / EP / ChP |
| Packaging | 500 g/bag; 1 kg/bag; 10 kg/bag; 10 kg/drum |
| Synonyms | SBE-β-CD; Sulfobutyl Ether Beta-Cyclodextrin; Betadex SBE Sodium |
| Assay | ≥99% |
| Bacterial Endotoxins | ≤10 EU/g |
| MOQ | 1 kg |

1. Uses
Betadex Sulfobutyl Ether Sodium is primarily used as a pharmaceutical excipient in:
● Ophthalmic (eye) preparations
● Nasal and pulmonary drug delivery systems
● Formulations of poorly water-soluble APIs, including antifungal, antiviral, anticancer, and cardiovascular drugs
2. Benefits
● Enhances solubility of poorly soluble drugs
● Improves stability by forming non-covalent inclusion complexes
● Reduces hemolysis and injection site irritation
● Decreases renal toxicity compared with conventional solubilizers
● Supports controlled drug release and improved bioavailability
● Masks unpleasant taste or odor of APIs
● High aqueous solubility and low toxicity for parenteral use

Over 20 Years of Manufacturing Experience
Xi’an Deli Biochemical Industry Co., Ltd. has extensive experience producing cyclodextrins and derivatives, ensuring high-quality products and reliable supply.
Stable Large-Scale Production
Annual capacity of 500 tons HPBCD and 200 tons Betadex SBE Sodium supports commercial and clinical applications.
Pharmaceutical-Grade Quality
Manufactured under strict quality management with Class D clean area, ensuring batch-to-batch consistency.
Advanced Laboratory Testing
Equipped with HPLC, GC, IC, CE, FTIR, and NMR, meeting USP, EP, ChP pharmacopeial standards.
Regulatory Support & Global Experience
Products registered with FDA; experienced in supporting global pharmaceutical markets.
Customer Technical Support
Professional guidance from formulation development to commercial supply.


Certificates and quality systems of Xi’an Deli Biochemical Industry Co., Ltd.

1. Are you a manufacturer or a trading company?
We are a professional manufacturer producing cyclodextrins and derivatives at our own facility in Xi’an, China.
2. What are your main products?
Hydroxypropyl Betadex (HPBCD) and Betadex Sulfobutyl Ether Sodium (SBECD), widely used as pharmaceutical excipients.
3. Do your products meet pharmacopeial standards?
Yes. All products comply with USP, EP, and ChP, with COA provided for each batch.
4. Do you supply injectable-grade materials?
Yes, with strict control of impurities, endotoxins, and batch consistency.
5. What is your production capacity?
HPBCD: 500 tons/year; Betadex SBE Sodium: 200 tons/year
6. Do you provide samples?
Yes, free samples are available for testing and formulation development.
7. Can you support long-term supply?
Yes, large-scale production and stable quality systems support commercial-scale supply.
8. What quality systems do you follow?
ISO 9001:2015, supported by advanced analytical equipment and dedicated QC lab.

 Betadex Sulfobutyl Ether Sodium CAS NO 182410-00-0
Betadex Sulfobutyl Ether Sodium CAS NO 182410-00-0 Sulfobutyl Ether Beta Cyclodextrin Sodium
Sulfobutyl Ether Beta Cyclodextrin Sodium Betadex Sulfobutyl Ether Sodium Injection Grade
Betadex Sulfobutyl Ether Sodium Injection Grade Betadex Sulfobutyl Ether Sodium SBECD Injectable Grade for Drug Formulations
Betadex Sulfobutyl Ether Sodium SBECD Injectable Grade for Drug Formulations Betadex Sulfobutyl Ether Sodium CAS 182410-00-0
Betadex Sulfobutyl Ether Sodium CAS 182410-00-0 DMF Betadex Sulfobutyl Ether Sodium 182410-00-0
DMF Betadex Sulfobutyl Ether Sodium 182410-00-0