


Wholesale SBE-β-CD Sulfobutylether Beta Cyclodextrin Sodium Injectable Pharmaceutical Excipient made in China from DELI. SBE-β-CD (Sulfobutylether Beta Cyclodextrin Sodium) is a highly water-soluble injectable pharmaceutical excipient used to enhance the solubility, stability, and safety of poorly soluble APIs through reversible inclusion complex formation.
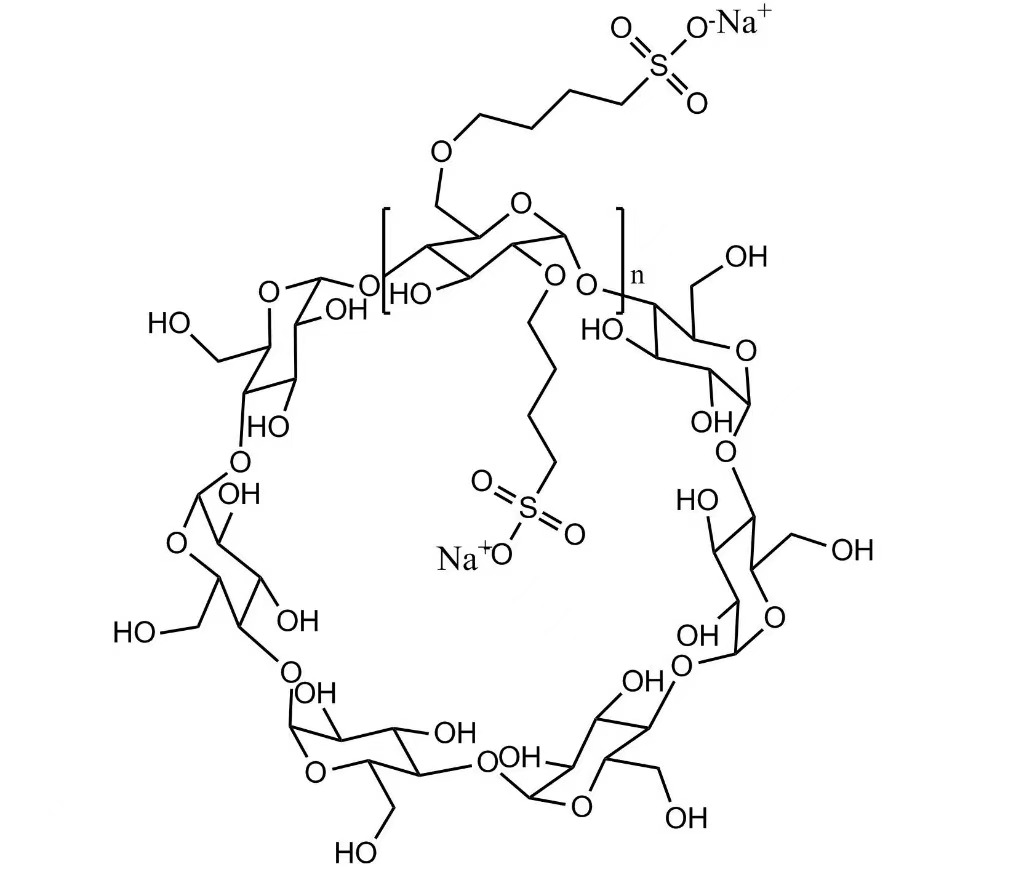
SBE-β-CD (Sulfobutylether Beta Cyclodextrin Sodium) is a highly water-soluble, anionic β-cyclodextrin derivative designed for injectable pharmaceutical applications. It forms stable, non-covalent inclusion complexes with active pharmaceutical ingredients (APIs), significantly improving the solubility, stability, and safety of poorly water-soluble drugs.
Due to its excellent biocompatibility, low hemolytic activity, and controlled toxicity profile, SBE-β-CD is widely used in parenteral drug delivery systems across multiple therapeutic areas.
SBE-β-CD enhances aqueous solubility of hydrophobic APIs while maintaining formulation safety. Compared with traditional solubilizing agents, it shows reduced renal toxicity and lower irritation at injection sites, making it suitable for repeated parenteral administration.
SBE-β-CD is primarily applied in injectable and sterile formulations, including intravenous, intramuscular, and subcutaneous dosage forms. It is commonly used in antiviral, antifungal, oncology, and cardiovascular drug products, where high solubility and formulation stability are critical.

| Chemical Name | Sulfobutylether Beta Cyclodextrin Sodium |
| CAS No. | 182410-00-0 |
| Appearance | White to off-white amorphous powder |
| Grade | Injectable Pharmaceutical Excipient |
| Solubility | Freely soluble in water |
| Assay | 95.0% – 105.0% (anhydrous basis) |
| Average Degree of Substitution (DS) | 6.2 – 6.9 |
| Bacterial Endotoxins | ≤ 10 EU/g |
| Standards | USP / EP |
| Shelf Life | 36 months |
Available packaging options include 500 g/bag, 1 kg/bag, 10 kg/bag, and 10 kg/drum. Customized packaging is available upon request for commercial supply.

All batches of SBE-β-CD are manufactured under a strict quality management system and undergo comprehensive analytical testing. Key quality parameters such as impurities, endotoxins, degree of substitution, and batch-to-batch consistency are tightly controlled.
Each shipment is accompanied by a Certificate of Analysis (COA) to ensure full traceability and regulatory compliance.

Xi’an Deli Biochemical Industry Co., Ltd. is a high-tech enterprise specializing in the research, development, and manufacture of cyclodextrins and their derivatives. Founded in 1999, the company has over 26 years of industry experience.
The company operates dedicated pharmaceutical excipient production lines with controlled clean manufacturing areas. Its quality management system has passed ISO 9001 certification, and core products are registered or filed with relevant regulatory authorities, including the FDA.

Xi’an Deli Biochemical has established large-scale commercial production capacity for SBE-β-CD, ensuring reliable long-term supply for global pharmaceutical customers.
Annual production capacity exceeds 200 metric tons, with a typical batch size of approximately 2.5 metric tons.

Q1: What is the main function of SBE-β-CD in injectable formulations?
A: SBE-β-CD primarily acts as a solubilizer and stabilizer, improving the aqueous solubility and safety of poorly water-soluble APIs through reversible inclusion complex formation.
Q2: Is SBE-β-CD suitable for repeated intravenous administration?
A: Yes. SBE-β-CD demonstrates low hemolytic activity and reduced renal toxicity compared with traditional solubilizing agents, making it suitable for repeated parenteral use.
Q3: Does your product comply with pharmacopeial standards?
A: Our SBE-β-CD complies with USP and EP requirements and is manufactured under a certified quality management system.
Q4: Can technical documentation be provided?
A: Yes. COA, specifications, and technical data are available upon request.
 Betadex Sulfobutyl Ether Sodium CAS NO 182410-00-0
Betadex Sulfobutyl Ether Sodium CAS NO 182410-00-0 Sulfobutyl Ether Beta Cyclodextrin Sodium
Sulfobutyl Ether Beta Cyclodextrin Sodium Betadex Sulfobutyl Ether Sodium Injection Grade
Betadex Sulfobutyl Ether Sodium Injection Grade Betadex Sulfobutyl Ether Sodium SBECD Injectable Grade for Drug Formulations
Betadex Sulfobutyl Ether Sodium SBECD Injectable Grade for Drug Formulations Betadex Sulfobutyl Ether Sodium CAS 182410-00-0
Betadex Sulfobutyl Ether Sodium CAS 182410-00-0 DMF Betadex Sulfobutyl Ether Sodium 182410-00-0
DMF Betadex Sulfobutyl Ether Sodium 182410-00-0